BioChemistry > EXAM REVIEW > Florida Atlantic University - BCH 3033 Biochem Final Exam StudyGuide. This document contains over 20 (All)
Florida Atlantic University - BCH 3033 Biochem Final Exam StudyGuide. This document contains over 200 Q&A, With Correct answers Indicated in bold.
Document Content and Description Below
Biochemistry Final Exam 1) Calculate the enthalpy of formation per mol of NH3: Bond energies: H2, 436k KJ/mol; N2, 945 KJ/mol, N-H, 391 KJ/mol a) -93 KJ b) -1080 KJ c) 1080 KJ d) 45 KJ e) -46... .5 KJ 2) Membrane structures are held primarily by? (OR membrane fs in a lipid bilayer are held together primarily by?) a) Hydrophobic interactions b) Covalent bonds c) Ionic Interactions d) All of the Above 3) Which system corresponds to the intracellular buffer in humans? a. H2PO4-/ HPO42-/ PO43- system b. CO2/HCO3/H2CO3 system c. All of Above d. None of Above 4) Which of the following has the best buffering capacity? a. 2M CH3COOH/ 2M CH3COO- b. 0.2M CH3COOH/ 0.2M CH3COO- c. 7M CH3COOH/ 7M CH3COO- 5) Which of the following statements is incorrect about the following amino acids? a. Phe and Ala are nonpolar b. His and Arg are basic c. Cys and Gly are polar d. Asp and Asn are acidic 6) Which of the following amino acids belong to the same group? a. leu, pro, ala, val b. his, arg, lys, glu c. gly, tyr, cys, pro d. asp, glu, asn, gln 7) Determine the amino acid sequence of the following oligopeptide from the experimental data below: 1. the amino acid composition is found to be ala, lys, phe, met, cys, trp 2. treatment with carboxypeptidase results in tryptophan and a peptide 3. CNBr treatment yields a tetrapeptide and a dipeptide 4. Trypsin digestion produces and amino acid and a pentapeptide with met on the amino end 5. Chymotrypsin digestion yields a dipeptide and a tetrapeptide a. trp-lys-met-cys-met-ala b. lys-ala-cys-phe-met-trp c. trp-ala-phe-cys-met-lys d. lys-met-cys-phe-ala-trp 8) What is the overall net charge of the following polypeptide at pH = 13? lys-lys-his-glu a. +2 b. 0 c. –2 d. 1 9) Place the following amino acids by increasing isoelectric points: his, ala, asp, lys, arg. a. asp, ala, lys, arg, his b. his, lys, arg, ala, asp c. arg, lys, ala, his, asp 10) Most non-basic and non-acidic isoelectric amino acids: a. give a basic solution in water b. give an acidic solution in water c. give a neutral solution in water 11) Which cannot be a component in an alpha helix? a. lys b. gly c. pro d. All are components 12) In beta keratin (composed of ala and gly) you will find: a. alternating ala & gly in opposite faces b. ala and gly on the same plane c. None of Above 13) The most abundant amino acids in collagen is: a. pro & gly b. gly & ala c. All of Above d. None of Above 14) Every third amino acid faces the crowded center of tropocollagen molecule. This amino acid is: a. gly b. pro c. Ala d. All of Above 15) All are globular proteins except: a. ribonuclease b. alpha-keratin c. transport protein 16) This is true of an alpha helix a. all carbonyl groups point in the same direction d. its has a pitch that is smaller than that of collagen b. the helix has axial polarity e. all of the above c. the helix is found in human keratin 17) Collagen is characterized by a. having long streches of gly-pro-pro/hyp c. has no lysine in its composition b. formula has repeats of abcdefg amino acids d. being made of alpha helices 18) Globular proteins are usually all EXCEPT a. Insoluble in water d. Hydrophobic side chains are exposed to the water b. Roughly spherical e. none are true c. Folded so that the hydrophobic amino acids are in the interior of the molecule 19) Membrane proteins differ from globular proteins in that a. membrane associated amino acids usually have polar side chains d. globular proteins are water insoluble b. membrane proteins are much more soluble in detergents than water e. All are true c. membrane proteins usually have more hydrophobic amino acids 20) Alpha keratin has a structure with a sequence that a. has 7 repetitive residues c. disulfide bridges connect the polypeptides b. the first and the fourth are nonpolar d. all of them 21) One of these is not found in collagen a. 3-hydroxyproline c. 5-hydroxylysine b. 4-hyrdroxyproline d. 3-hydroxy-glycine 22) The separation between two proximal beta strands of a beta sheet is wider in the? a. paralell arrangement c. the have the same separation b. antiparalell arrangement d. none of them 23) The unique composition of collagen is accommodated in a structure called a(n): a. -pleated sheet. d. coiled coils. b. triple helix. e. all are true c. helix-turn-helix motif 24) The rate constant for a third order reaction has the unit: a. M-1 b. M-1-s-1 c. s-1 d. M-2-s-1 25) For the above graph, what is the Km for the uninhibited reaction? a. 0.05 b. 0.1 c. 0.01 d. 0.5 26) What is the Vmax for the completely inhibited reaction? a. 0.25 b. 10 c. 0.40 d. 0.35 27) Know how to calculate Kcat 28) What is the enzyme efficiency? 29) Coenzymes are derived from a. vitamins b. globular proteins c. Membrane Proteins d. Beta- Sheets 30) Viagra, asprin, and lipitor are all: a. enzyme inhibitors b. enzyme activators c. Both inhibitors and activators d. None 31) Enzyme specificity is best explained in the terms of: a. lock and key hypothesis b. proenzyme activation c. induced fit hypothesis d. pos-tranlational modification model 32) Enzymes are regulated by: a. zymogen generation b. covalent modification c. accumulation of products d. effectors binding at the allosteric site e. all the above 33) Enzymes which catalyzes the reaction below is classified as: Alcohol + NAD+ aldehyde + NADH + H+ a. dehydrogenase (correct answer) 34) Chymotrypsin: a. zymogen generation b. effects binding at the allosteric site c. accumulation of the product d. covalent modification 35) Enzymes covalently regulated by 2 different enzymes are: a. interconvertable enzymes b. competitive enzymes c. noncompetitive enzymes d. None of the above 36) Lactate dehydrogenase combines 2 subunits in variable ratios. This is an example of a: a. isoenzyme b. interconvertable enzyme c. competitive enzyme d. holoenzymes 37) Glycogen phosphorylase b is activated by: a. AMP b. ATP c. Caffeine d. Glucose-6-P 38) All of the following are correct statements about enzyme regulation except: a. Enzymes can be inhibited by the products they produce b. Enzymes can be inactivated by the addition of a functional group c. Coenzyme and substrate availability can regulate enzyme reaction rate d. The activity of an enzyme is covalently affected by allosteric regulators 39) All are characteristic of allosteric enzymes except: a. Effectors may show stimulatory or inhibitory activity b. They have multiple subunits c. They obey michaelis-menten kinetics d. The regulatory effect is by altering conformation and interaction of subunits e. Binding one subunit impacts binding substrate to other subunits 40) When binding one equivalent of substrate to an allosteric protein enhances the binding of additional equivalent of S to the same protein molecule, it is termed an: a. Negative heterotropic effector b. Positive homotrophic effector c. Posititive heterotropic effector d. Negative homotropic effector 41) The function of glycogen phosphorylase is: a. The conversion of glucose-1-phospate to glucose-6-phospate b. To break down ATP c. To catalyze the phosphorolysis of glucose-1-phospate from glycogen molecules d. To inhibit the production of glucose-1-phosphate 42) Which of the following peptides would absorb the most light centered at 280nm? a. trp-tyr-thr-lys-phe b. lys-ala-trp-trp-ala c. thr-thr-phe-tyr-lys d. tyr-thr-val-thr-thr 43) The amino acid residues which are directly involved in the sugar-protein linkage in glycoproteins are: a. gly, thr & ser c. lys, his & glu b. gln, ser & tyr d. asn, thr & ser 44) The thermodynamic driving force for passive diffusion depends on a. the thickness of the membrane c. the hydrophobic character of the species b. the chemical potential gradient d. the permeability coefficient of the species 45) What is not true about cell membranes? a. made of lipid bilayer c. composed of glycerophospholipids, proteins, cholesterol b. have a wide range of composition d. same composition in the inner and outer face 46) Which of the following transport processes does not require a transport protein? a. passive diffusion c. symport transport b. active transport d. facilitated transport 47) The rate of passive diffusion depends on a. the chemical potential gradient c. the partition coefficient for the species b. the thickness of the membrane d. all of the above 48) An aspect which is not a characteristic of the Na+,K+ATPase is a. it is an electrogenic pump c. its function is to pump Na+ ions out of and K+ ions into the cell b. the process requires the hydrolysis of ATP and the subsequent hydrolysis of pyrophosphate for each transport event d. the ATPase activity is associated with the cytoplasmic side of the pump 49) The ATPase which is responsible for maintaining the greatest ion gradient is the a. Na+-/K+ATPase c. Ca++‚ ATPase b. erythrocyte anion transporter d. H+,K+ ATPase 50) A transport system which can be described as a symport system is a. Na+,K+ATPase c. glucose permease b. H+,K+ ATPase d. Ca++‚ ATPase 51) Which statement is incorrect about the mechanism of the Na+-/K+ pump? a. The pump phosphorylates itself when ATP, Mg++ and Na+ are bound c. The cartiotonic steroid, digitalis, inhibits the pump by binding to a site on its extracellular face. b. One mole of ATP is hydrolyzed for every three moles of Na+ transported out of the cell. d. The phosphorylated pump has a higher affinity for K+ than for Na+. 52) Give the name of the sugar in the figure below: a. sialic acid c. galactosamine acetate b. glucosamineacetate d. heparin acetate 53) Which of the following is not an aldohexose? a. glucose c. fructose b. allose d. mannose 54) Which of the following cannot undergo mutarotation? a. glucose c. fructose b. ribose d. trehalose 55) Which of the following disaccharides contain a beta-1,4-glycosidic bond? a. lactose c. sucrose b. maltose d. glucose 56) What is the name of the structure below? a. galactose-beta-1,4-fructose c. mannose-beta-1,4-fructose b. glucose-alpha-1,4-fructose d. fructose-alpha-1,2-glucose 57) Aldohexoses prefer the following type of structure? a. pyranose ring c. furanose ring b. linear structure d. none of the above 58) Which of the following characteristics is not associated with glycogen? a. It is a branched polymer of glucose c. The enzyme, cellulase can hydrolyze o-glycosidic linkages in this polymer. b. It contains more alpha-1,4-glycosidic bonds, than alpha-1,6-glycosidic bonds d. It is an energy storage molecule rather than a structural molecule 59) Which molecule is not a glycosaminoglycan? a. heparin c. keratan sulfate b. chondroitin-4-sulfate d. chitin 60) The following structure is that of? a. cellulose c. amylose b. cellobiose d. sucrose 61) The process shown below is called: a. steroisomerization c. anomerization b. Alpha, beta inversion d. mutarotation 62) Oxidation of allose on C6 will produce? a. allonic acid c. alluronic acid b. allaric acid d. allopuric acid 63) Structure below is that of an: a) Steroid Molecule b) Spingolipid c) Ceraboside d) None of the above 64) Sphingomyelins are found preferentially in: a. skeletal muscle tissue b. nerve sheets c. heart membrane d. any tissue 65) Enzymes that can change membrane face composition are called: a. flippases b. synthetases c. lipases d. porins 66) One of the fatty acids below is not good for our cardiovascular health a. linoleic b. oleic c. palmitic d. arachidonic 67) What does the cartoon below represent? a. action of ionophore c. symport protein b. flippase d. antiport protein 68) The difference between starch and glycogen is a. starch has a and glycogen b glycogen glycosidic bonds b. starch has only 1,4 glycosidic linkages c. starch has 1,6 glycosidic linkages and glycogen does not d. glycogen is more branched than starch 69) Those enzymes that are covalently regulated by two difference enzymes are a. isoenzymes b. allosteric enzymes c. interconvertible enzymes d. michaelis menten enzymes 70) Lactate dehydrogenase combines two subunits in variable ratios to control its activity a. holoenzymes b. isozymes c. proenzymes d. zymogen e. heteroterameric 71) A negative heterotropic effector of glycogen phosphorylase is a. ATP b. Phosphate c. Nicotine d. AMP 72) One of these has enzymatic activity a. genomic DNA d. small ribosomal subunit b. siRNA e. large ribosomal subunit c. mRNA 73) The Klenow fragment has the following activities a. polymerase and 3’ exonuclease c. polymerase and 5’ exonuclease b. 3’ and 5’ exonuclease d. 3’and 5’ exonuclease plus polymerase 74) Gyrase is a a. type I topoisomerase b. helicase c. type II topoisomerase d. ligase 75) Analysis of a cell line that rapidly transforms into a tumor cell line demonstrated an increased mutation rate within the cell. Further analysis indicated that there was a mutation in the DNA polymerase enzyme that synthesizes the leading strand. This inactivating mutation is likely to be in which of the following activities of this DNA polymerase. a. 5’-3’ Exonuclease activity d. Uracil-DNA glycosylase activity b. 5’-3’ polymerase activity e. 3’-5’ exonuclease activity c. Phosphodiester bond making capability EXTRAS (FOUND IN MULTIPLE FINAL EXAMS SO STUDY THESE TOO) 76) The common activities of DNA pol I and Pol III are a. Polymerase, 5’ and 3’exonuclease c. Polymerase and 5’exonuclease b. Polymerase and 3’exonuclease d. Polymerase only 77) Of the polymerase in E.Coli, the most processive is a. Pol I c. Pol III b. Klenow d. None of the above 78) The “proof reader” activity of Pol 1 is its a. Polymerase activity c. 3’polymerase b. 5’exonuclease activity d. Endonuclease activity 79) The “editor” activity of Pol I a. Polymerase activity c. 3’ exonuclease activity b. 5’exonuclease activity d. Endonuclease activity 80) The enzyme which removes the RNA primer from the Okazaki fragments is a. Pol III c. Primase b. Klenow fragment d. Pol 1 81) The polymerase which plays a similar role as that of Prokaryotic pol III in eukaryotic replication is a. DNA polymerase alpha c. DNA polymerase delta b. DNA polymerase beta d. DNA polymerase gamma 82) In DNA polymerase III, the sliding clamp is the a. Alpha subunit c. Theta subunit b. Beta subunit d. Epsilon subunit 83) If you wanted to silence a gene, you would use: a. mRNA b. siRNA c. tRNA d. rRNA 84) In order to assemble eukayrotes ribosomes the following metal is needed: a. Ca ++ b. Mg ++ c. Na+ d. Fe+++ 85) In order to repair mutant U in DNA, nature uses this base as a marker a. adenine b. guanine c. thymine d. cytosine 86) How many helical turns (the linking number) are found in a relaxed circular DNA of 4500 bp? a. 450 b. 400 c. 550 d. 1000 87) In the Sanger’s DNA sequencing method, what is the reason for the stop of chain elongation? a. dideoxynucleotide triphophate b. primer strand c. the template strand has been synthesized which then stops in it d. one of the dNTPS 88) Nick translation is preformed by: a. helicase b. primase c. Polymerase 1 d. Polymermase III 89) Poly A polymerase is used in: a. attaching a run of A’s to mRNA on the 3’ end b. making prokaryotic DNA c. All of them d. the initiation of RNA synthesis at purine site 90) The site where RNA polymerase binds is the a. Promoter c. The OriC site b. The starting site d. The template 91) Transcription is thought to generate a. Positive supercoils behind the transcription bubble c. Negative supercoils in front of the transcription bubble b. Positive supercoils in front of the transcription bubble d. A relaxed DNA during the transcription process 92) A strong promoter is one that a. Is GC rich c. Has closest sequence to consensus b. Is AT rich d. Has weakly bound open complex 93) Which statements are correct about enhancer sequences in eukaryotes? a. Occur within a gene c. The can be in either orientation b. The can occur either 3’or 5’ to the gene d. They are promiscuous 94) The co-inducer in negative control of the lac operon is a. CAP c. Glucose b. Lactose d. Repressor 95) The Klenow fragment of E. coli DNA polymerase I can be used to add nucleotides to a DNA with staggered ends if the ends a. Are recessed with 5’-phosphates c. Are extended with 5’-phosphate b. Are extended with 3’-OHs d. Are recessed with 3’-OHs 96) The enzyme that extends the 3’ end at the replication primer gap in eukaryotes is a. Polymerase c. Pol delta b. Telomerase d. Contrahelicase 97) Under which of the following conditions does the melting temperature for the denaturation of duplex DNA decrease? a. higher salt concentration in the buffer b. higher GC content in the DNA c. longer DNA fragments d. higher AT content in the DNA 98) The sense sequence is read from a. 3’-5’ c. 5’-3’* b. From C terminus to N terminus d. Either way 99) Which of the following characteristics is associated with the B form of DNA? a. Both C and D c. It has major and minor grooves b. The number of bases per turn is 12 d. The number of bases per turn is 10 100) A feature which is distinctive to Z-DNA is a. A left handed helix c. It is found in vivo b. It has 12 bases per turn d. All of the above 101) Which of the following double stranded DNAs may convert to cruciform structure? a. ATGCATGCATGCATGC TACGTACGTACGTACG c. CATTAAGCAGTGCTTAAGA GTAATTCGTCACGAATTCT b. ATATATATGCGCGCGCGC TATATATACGCGCGCGCG d. AAAAAAAAACCCCCCCC TTTTTTTTTGGGGGGGG 102) Which E. coli enzyme has the greatest frequency of incorporating the incorrect nucleotide into its nucleic acid polymer? a. RNA polymerase c. DNA polymerase I b. DNA polymerase III d. Klenow fragment from DNA polymerase I 103) The 5 subunits of prokaryotic RNA polymerase form the a. RNA polymerase c. Close complex b. Open complex d. Core polymerase 104) Which statement is incorrect about the structure of E. coli chromosomal DNA? a. The DNA is primarily in the B-helix c. The DNA exists as a right-handed double helix b. The DNA is circular and supercoiled d. The DNA is one large linear piece about 4000 kb in length 105) The E. coli promoter is recognized by RNA polymerase using a. The beta subunit c. The alpha subunit b. The sigma subunit d. The beta primer subunit 106) The Pribnow box is also called a. The -35 consensus sequence c. The TATA box b. The polymerase d. The TTGACA box 107) By what mechanism is glucose transported from the intestine into mucosal cells? a. facilitated diffusion b. in exchange for Na+ c. in symport with Na+ d. primary active transport 108) Which lipid is least likely to be found in the plasma membrane of erythrocytes? a. triglyceride b. sphingomyelin c. ganglioside d. cholesterol 109) Which of the following is NOT true of the properties of water? a. Water is a polar molecule. b. Water is hydrogen-bond donor and acceptor. c. Water has low dielectric constant. d. Water has potential to form 3 or 4 hydrogen-bonds per molecule. 110) Which of the following statements is correct for hemoglobin and oxygen transport? a. The oxygen binds to the proximal histidine residue of the globin chain. b. Bonding of carbon dioxide to hemoglobin molecules increases the binding of oxygen. c. Hemoglobin binds more oxygen at higher [2, 3-BPG] concentrations. d. The binding of each O2 molecule to hemoglobin increases its affinity for the next O2. a) The promoter site b) A mutation in the Lac Z gene would result c) Negative control of the lac operon is achieved by d) Zn DNA motif is found in e) The protein reading of N-terminus to C- terminus is same as f) A consensus sequence is TATAAT. Which of the following promoters would be the most efficient? g) The Pribnow box is a h) Which of the following enzymes of DNA pol I o pol III is most abundant in E. coli? i) In Eukaryotes, mRNA is made by: j) Which of these eukaryotic enzymes has unlimited processivity? k) Since the heptapeptide repeats in the carboxyl terminal domain in the large subunit of RNA polymerase II can be phosphorylated, the repeats must contain a. Serine c. Histidine b. Aspartic acid d. Tryptophan l) A group of genes transcribed at the same time and controlled by one protein is called an Answer: m) Poly A polymerase is used for n) In eukaryotes the structure genes for biochemical pathways o) For the DNA polymerase to be able to elongate the hn RNA of the carboxyl terminal domain in the large subunit it must be Answer: p) In supercoiled B-DNA which is false? q) In eukaryotes, there are many more proteins than genes. This is explained by r) Positive control in the lac operon is achieved by? s) Ratio of pure DNA is 260/280= 1.8 t) Ratio of pure RNA is 260/280= 2.0 u) Intracellular pH is maintained primarily by the ________ and __________ buffer systems, and the extracellular pH by the ____________ buffer system. Answer: HPO42-/H2PO4-; histidine; HCO3-/H2CO3 Extra Questions he could ask: 1. Prokaryotes are? Polycistronic a. NOT circular genes, and... 2. A ribose sugar and a base is a? nucleoside 3. Which is an example of a purine? Guanine 4. When thymine and ribose is combined, you get? Thymidine 5. In nature DNA uses this molecule to detect mutation. Thymine 6. In pure DNA absorbance ratio is? 260/280 = 1.8 a. If question asked pure RNA? 260/280 = 2.0 7. The rule that states that A:T + G:C =1? Chargaff 8. Sequence pAATTCCGG complementary strand? 5’CCGGAATT3’ 9. Sense strand reads? 5’-3’ 10. Which of the following is not used in protein synthesis? hnRNA 11. The following structure relates to the next two questions. What is this structure? ssDNA a. If it contained uracil, it would be ssRNA! 12. Exonuclease 3-“b” cutter gives you? pGATAp, C a. This cuts at B on the outside of the phosphate bond leaving C with nothing attached 13. Gel electrophoresis DNA sequence with primer ATAT? Original sequence after reading from bottom up, then reversed to be read in 5’-3’ direction is 5’CTCC.......ATAT3’ 14. Which of the following gives a strong cruciform? a. Answer A with all the G;C since these are tighter bonds b. Sucker choice was C since it was on all the old exams and contained mostly A:T 15. Diagram of cyclic AMP. This structure most likely functions as? Cell messenger (cAMP is a secondary messenger) a. NOT all of the above (energy transfer, capping 5’ ends) 16. Diagram of melting points where on sigmoid curve is more directed to right of chart. By inference the melting points for the structure one the right would? All of the above a. Contain more G:C b. More H-bonding c. Form stronger cruciform structure 17. Two species DNA are melted and then allowed to cool. Which of the following is true about this experiment? a. The higher the melting point of the two species, the closer related they are. b. The DNA strands are the same in mixture before melting. Big debate on grammatical inconsistency on this answer choice since most people picked all of the above because of the “ambiguous” or bad wording. c. The lower the melting temperature the more related the species are d. All of the above 18. The rate at which polymerase can add nucleotides and detach? 19. DNA Polymerase does not have which of the following: 20. Nick translation is possible through? 21. Large Klenow fragment is made with? 22. Common characteristics shared by Pol I and Pol III is? 23. Which is the “editor”? 3’ 24. Prokaryotic DNA Pol III is analogous to? 25. DNA Pol III replicates the lagging strand by using? 26. Tus proteins are a type of? 27. Mitochondrial DNA is made by? 28. Open 3’end primer gap is filled by? T 29. Reverse transcription has all of the following features except? a. Just the 3 enzyme activities RNA directed DNA Pol, RNAse H activity to degrade complementary RNA strand, DNA directed DNA Pol 30. Which of the following uses a tRNA primer? 31. Density gradient for DNA in a conservative model? a. One DNA strand is replicated making zero generation 1:1 ratio, but all generations after that will be 1:3 because that new second strand will make a duplicate of the original also so three new strands to one original strand. 32. Unlimited processing in eukaryotes? 33. A gene is a DNA sequence that codes for? (protein, tRNA, rRNA) 34. Eukaryotic mRNA is made by? 35. Which of the following is a complement of mRNA? , because sense is the lagging strand OR the mRNA 36. The site that RNA polymerase binds is? OriC is a choice also, but this would require it to say replication or E. Coli 37. Close promoter complex is? 38. A strong promoter sequence is? 39. rHO factor is a? 40. Which of the following is correct about enhancer sequences? a. Can occur within a gene b. Bidirectional c. Promiscuous d. Up or downstream 41. A group of genes that is transcribed by one protein is 42. E. Coli produces abundant amount of lactose and mutated B-Galactidose. Which of the following is responsible? 43. Increase in gene expression is a form of? 44. IPTG is unique in that? 45. CAP activation requires? 46. Zn-finger motif is found in? 47. Poly-A? a. NOT all of the above. Makes DNA synthesis, etc 48. TATA, CAAT, GC box are? a. Found upstream from initiation site b. CAAT means its a strong promoter c. Modulator components of genes/DNA 49. RNA Pol II is responsible in? 50. Pribnow box is found? 51. What type of inhibitor reversibly binds to the active site of the enzyme? 52. TFDII is a protein complex that binds to the? 53. What type of inhibitor irreversibly binds to the active site of the enzyme? 54. A heterotropic effector that activates an enzyme will have what effect on the sigmoidal kinetic plot (V vs. [S])? 55. D-glucose and D-galactose are? 56. The genetic code is said to be degenerate. This means that: 57. The Henderson-Hasselbalch equation shows that: 58. Vitamin C is needed to make 59. The extracellular buffer in humans is a. CO2/H2CO3/HCO3- system b. H2PO4-/HPO42- system c. The anserine system d. The acetate system [Show More]
Last updated: 1 year ago
Preview 1 out of 26 pages

Reviews( 0 )
Recommended For You
Chemistry> EXAM REVIEW > CHEM115 MidTerm Exam Review Azusa Pacific University - Correct Answers Indicated with Feedback. (All)
CHEM115 MidTerm Exam Review Azusa Pacific University - Correct Answers Indicated with Feedback.
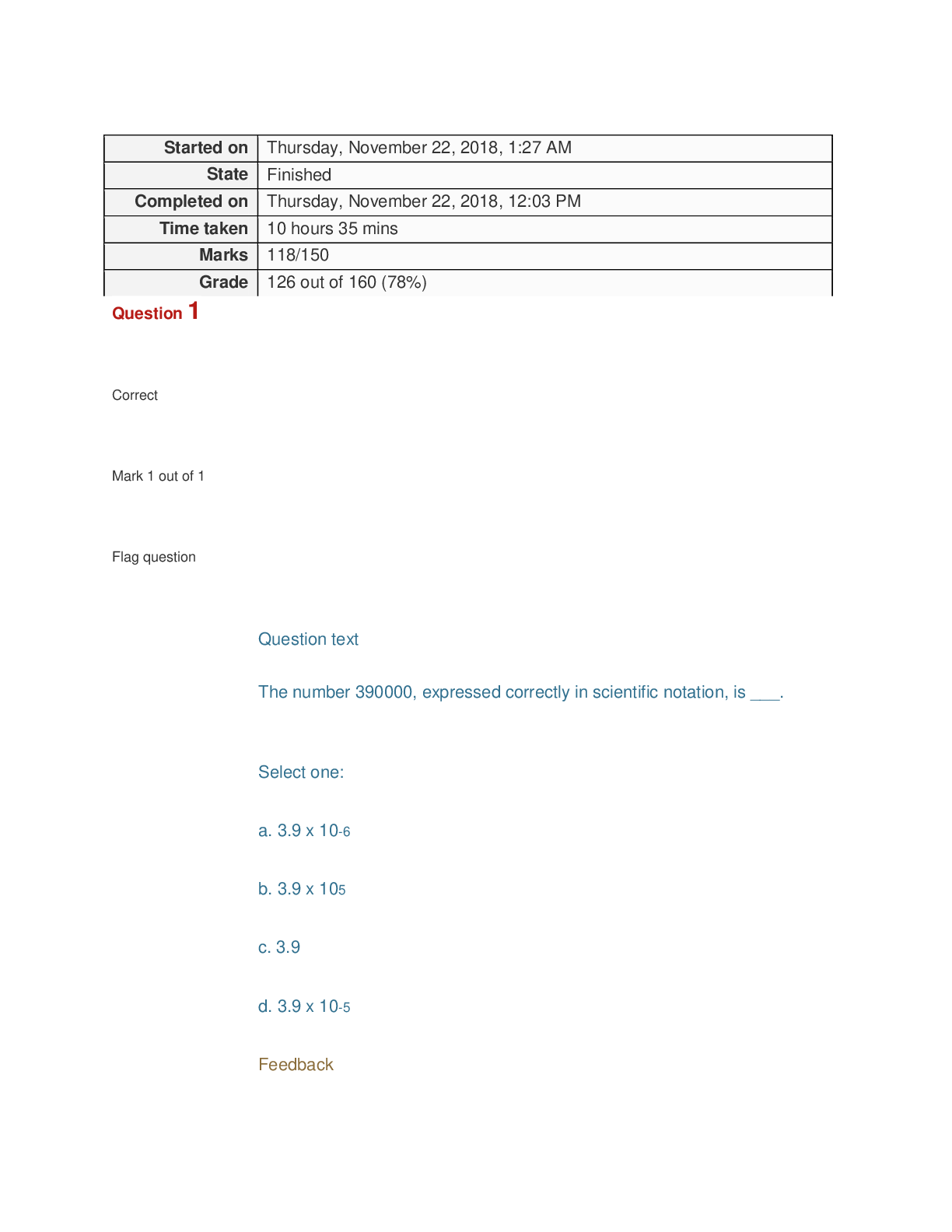
Grade 126 out of 160 (78%) Question 1 Correct Mark 1 out of 1 Flag question Question text The number 390000, expressed correctly in scientific notation, is ___. Select one: a. 3.9 x 10-6 b. 3...
By Kirsch , Uploaded: Feb 01, 2021
$15
*NURSING> EXAM REVIEW > ATI RN Maternal Newborn Online Practice 2019 A [Correct Answers indicated after each Question] (All)

ATI RN Maternal Newborn Online Practice 2019 A [Correct Answers indicated after each Question]
Contains 60 questions with the correct answers listed under each question A nurse is caring for a client who has uterine atony and is experiencing postpartum hemorrhage. Which of the following actio...
By TipsMaster01 , Uploaded: Aug 24, 2021
$12
BioChemistry> EXAM REVIEW > Florida Atlantic University - BCH 3033Biochem Final Exam StudyGuide. This document contains over 200Q&A, With Correct answers Indicated in bold. (All)
.png)
Florida Atlantic University - BCH 3033Biochem Final Exam StudyGuide. This document contains over 200Q&A, With Correct answers Indicated in bold.
Biochemistry Final Exam 1) Calculate the enthalpy of formation per mol of NH3: Bond energies: H2, 436k KJ/mol; N2, 945 KJ/mol, N-H, 391 KJ/mol a) -93 KJ b) -1080 KJ c) 1080 KJ d) 45 KJ e) -46 .5 KJ 2)...
By Tutor Frankline , Uploaded: Jul 23, 2021
$11
BioChemistry> EXAM REVIEW > Florida Atlantic University - BCH 3033Biochem Final Exam StudyGuide. This document contains over 200Q&A, With Correct answers Indicated in bold. (All)
.png)
Florida Atlantic University - BCH 3033Biochem Final Exam StudyGuide. This document contains over 200Q&A, With Correct answers Indicated in bold.
Biochemistry Final Exam 1) Calculate the enthalpy of formation per mol of NH3: Bond energies: H2, 436k KJ/mol; N2, 945 KJ/mol, N-H, 391 KJ/mol a) -93 KJ b) -1080 KJ c) 1080 KJ d) 45 KJ e) -46 .5 KJ 2)...
By A+ Grades , Uploaded: Jul 23, 2021
$10
BioChemistry> EXAM REVIEW > Questions and Answers > Florida Atlantic University - BCH 3033biochem si exam 2 review. Handwritten (All)
Questions and Answers > Florida Atlantic University - BCH 3033biochem si exam 2 review. Handwritten
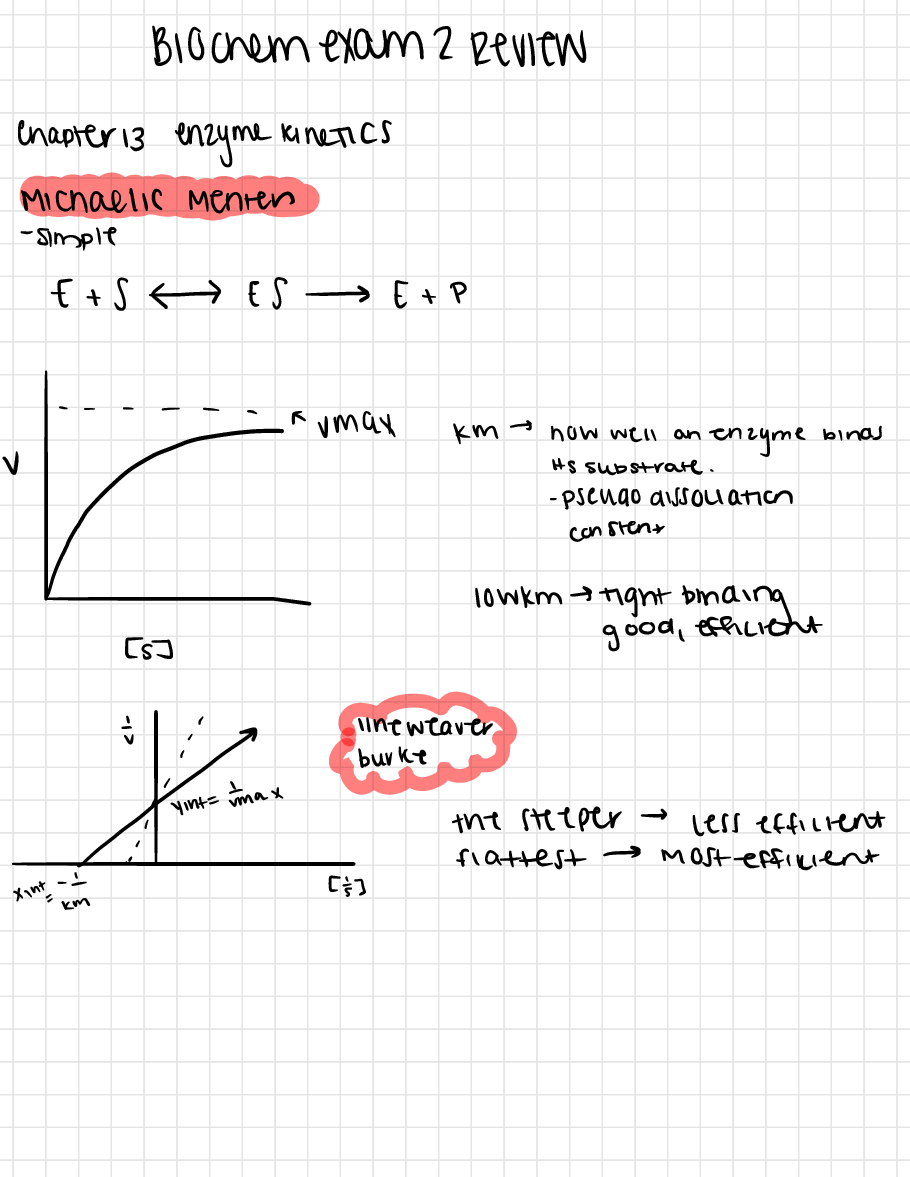
Questions and Answers > Florida Atlantic University - BCH 3033biochem si exam 2 review. Handwritten
By Kirsch , Uploaded: Feb 24, 2021
$13
Chemistry> EXAM REVIEW > CHEM 115 Final. 135 Questions and Answers. All correct Answers Indicated. (All)

CHEM 115 Final. 135 Questions and Answers. All correct Answers Indicated.
CHEM 115 Final Question 1 Correct Mark 1 out of 1 Flag question Question text MEK is the abbreviation for methyl ethyl ketone. It is the common name for a solvent that is sold at hardware and...
By Kirsch , Uploaded: Feb 01, 2021
$13
*NURSING> EXAM REVIEW > NURS 6501 Midterm Exam Review Guide (Weeks 1-6. Compilation in 99 Pages) (All)

NURS 6501 Midterm Exam Review Guide (Weeks 1-6. Compilation in 99 Pages)
Nurs 6501 Midterm Exam Review Guide (Weeks 1-6) Cellular Processes and the Genetic Environment 1. Describe cellular processes and alterations within cellular processes. 2. What is the impact of t...
By SuperSolutions© , Uploaded: Nov 24, 2020
$15
*NURSING> EXAM REVIEW > NUR2063 / NUR 2063 Essentials of Pathophysiology Exam Review Latest Update Rasmussen College (All)

NUR2063 / NUR 2063 Essentials of Pathophysiology Exam Review Latest Update Rasmussen College
NUR 2063 Essentials of Pathophysiology Exam Review 1. A potentially lethal condition in which there is an acute elevation of circulating thyroid hormones is called _______________ - ANS: Thy...
By quiz_bit , Uploaded: Oct 28, 2020
$12
*NURSING> EXAM REVIEW > Multidimensional Care (MDC) 1 MDC EXAM 2 REVIEW -. (Rasmussen College) (All)

Multidimensional Care (MDC) 1 MDC EXAM 2 REVIEW -. (Rasmussen College)
MDC EXAM 2 REVIEW 11:19:19 1. The effects of Immobility a) Interventions that improve flexibility • P.R.E.P.(Perform passive ROM, Reposition Q2HR, Encourage independent activity as much as possible...
By SuperSolutions© , Uploaded: Dec 11, 2020
$11
*NURSING> EXAM REVIEW > NUR 2356 / NUR2356 Multidimensional Care I (MDC I) Exam 2 Review. Rasmusssen College (All)

NUR 2356 / NUR2356 Multidimensional Care I (MDC I) Exam 2 Review. Rasmusssen College
1. What is a function of the musculoskeletal system? -Assist with movement 2. As a nurse you know that during aging, a normal musculoskeletal change would be? -A patient that came to the cli...
By nurse_steph , Uploaded: Nov 15, 2020
$11
Document information
Connected school, study & course
About the document
Uploaded On
Feb 24, 2021
Number of pages
26
Written in
Additional information
This document has been written for:
Uploaded
Feb 24, 2021
Downloads
1
Views
148






