Health Care > QUESTIONS & ANSWERS > MCB 450 – Spring Exam I University of Illinois, Urbana Champaign MCB 450 (All)
MCB 450 – Spring Exam I University of Illinois, Urbana Champaign MCB 450
Document Content and Description Below
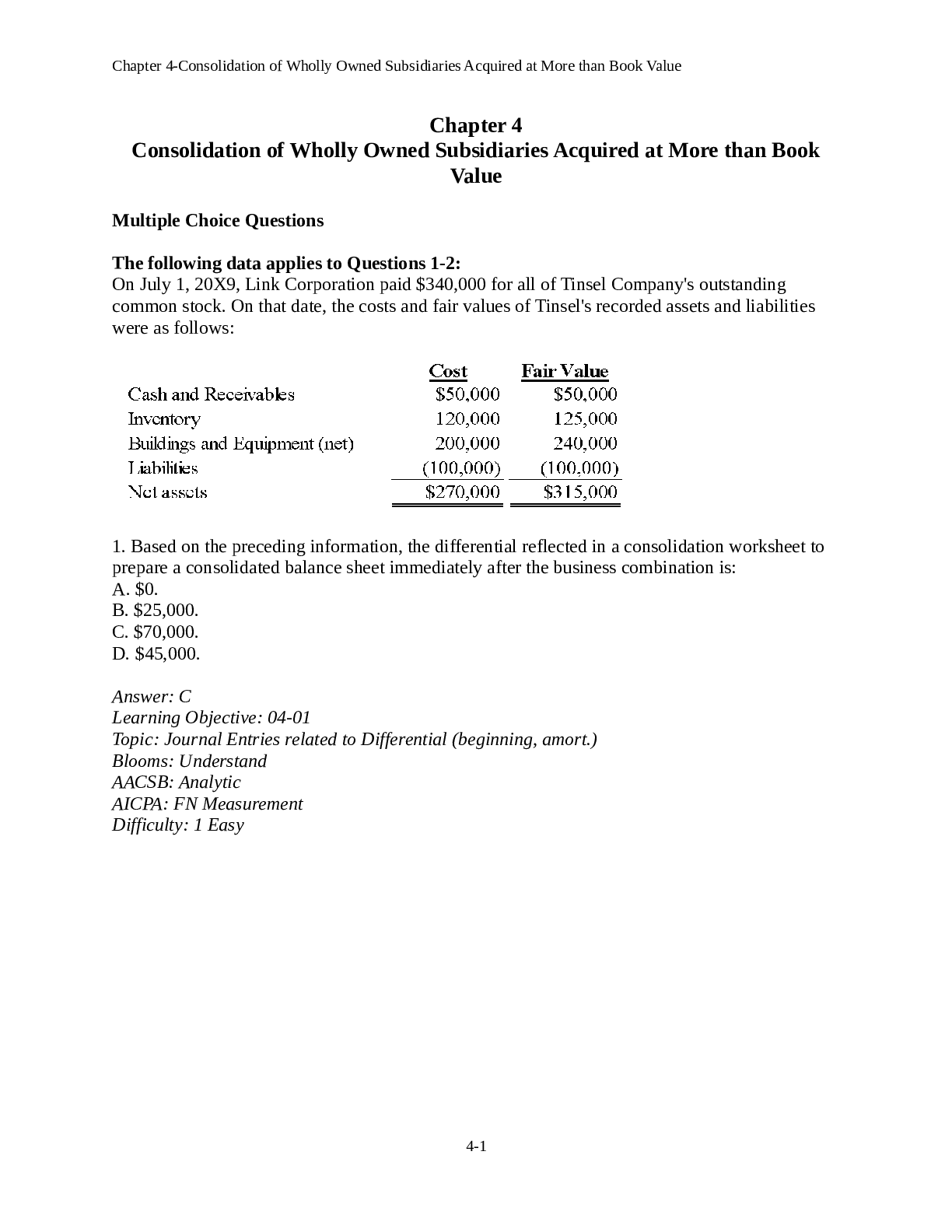
MCB 450 – Spring 2014 Exam I Form A Choose the BEST answer from the available choices. Only nongraphing calculators are permitted for use on this exam. Section 1 32 Multiplechoice Quest... ions. Each question is worth 4 points. (total of 128 possible points). 1. In the Central Dogma, RNA synthesis is to protein synthesis as: a. Replication is to transcription b. Translation is to transcription c. Translation is to reverse transcription d. Transcription is to translation e. Replication to translation 2. Lipids aggregate to form bilayers because: a. Some lipid molecules are hydrophobic and other lipid molecules are hydrophilic. b. Aggregation of hydrophobic molecules decreases their entropy c. Aggregation of hydrophobic molecules increases their entropy d. Aggregation of hydrophobic molecules decreases the entropy of surrounding water e. Aggregation of hydrophobic molecules increases the entropy of surrounding water 3. Hydrogen bonds can occur when the hydrogen is covalently bonded to atoms like 2 nitrogen and oxygen (hydrogenbound donors). What property of nitrogen and oxygen is important for this? a. Atomic mass. b. Ionizability. c. Hydrophobicity. d. Electronegativity. e. Greater number of unpaired electron. 4. What is the concentration of hydroxide ion in an aqueous solution with an H+ concentration of 2 × 10 5 M? a. 2 × 10 9 M b. 2 × 10 19 M c. 2 × 10 19 M d. 5 × 10 10 M e. Cannot calculate from the information given. 5. What is the approximate pH of a 0.10 M solution of a weak acid that has a Ka of 5 × 10 5 M? a. 2.7 b. 5.3 c. 4.8 d. 11.3 e. Cannot determine from the information given. 6. For a weak acid with a pKa = 6.5, the effective buffering range is usually considered to be: a. pH 6 to pH 7. b. pH 6.4 to pH 6.6. c. pH 5.5 to pH 7.5. d. pH 2.0 to pH 5.5. e. dependent on the molarity of the acid. 2 NAME:______________________________ NETWORK ID:___________________ 7. According to the HendersonHasselbalch equation, when the concentrations of proton acceptor and proton donor are the same, then: a. the carboxylic acid is totally neutralized. b. only salt forms are present. c. pH = pKa. d. pKa = log[proton acceptor]/[proton donor]. e. the pH value is 1 unit greater than pKa. 8. What would be a good way to increase by 10 fold the buffering capabilities of a weak acid solution? a. Increase its pKa by 1 unit. b. Adjust the pH of the buffer to 10 units above its pKa value. c. Adjust the pH of the buffer to 1 unit below its pKa value. d. Increase its Ka by 1 fold. e. Increase the concentration of the weak acid buffer by 10 fold. 9. How do the pKa values of an amino terminal group compare when the amino acid is free versus when it is in a polypeptide chain? a. The pKa of the terminal amino group is independent of whether the amino acid is in a polypeptide chain or is free. b. The pKa of the terminal amino group is usually lower in the free amino acid. c. The pKa of the terminal amino group of an amino acid in the middle of a polypeptide chain may be lower or higher than on free amino acids depending in differences in their microenvironments. d. The pKa of the amino group is usually higher in a polypeptide chain. e. The pKa of the am [Show More]
Last updated: 1 year ago
Preview 1 out of 14 pages

Reviews( 0 )
Document information
Connected school, study & course
About the document
Uploaded On
Feb 09, 2023
Number of pages
14
Written in
Additional information
This document has been written for:
Uploaded
Feb 09, 2023
Downloads
0
Views
60


.png)



.png)


.png)








 (2).png)
.png)