Chemistry > QUESTION PAPER (QP) > June 2021 QP - Paper 3 OCR (A) Chemistry A-Level. (All)
June 2021 QP - Paper 3 OCR (A) Chemistry A-Level.
Document Content and Description Below
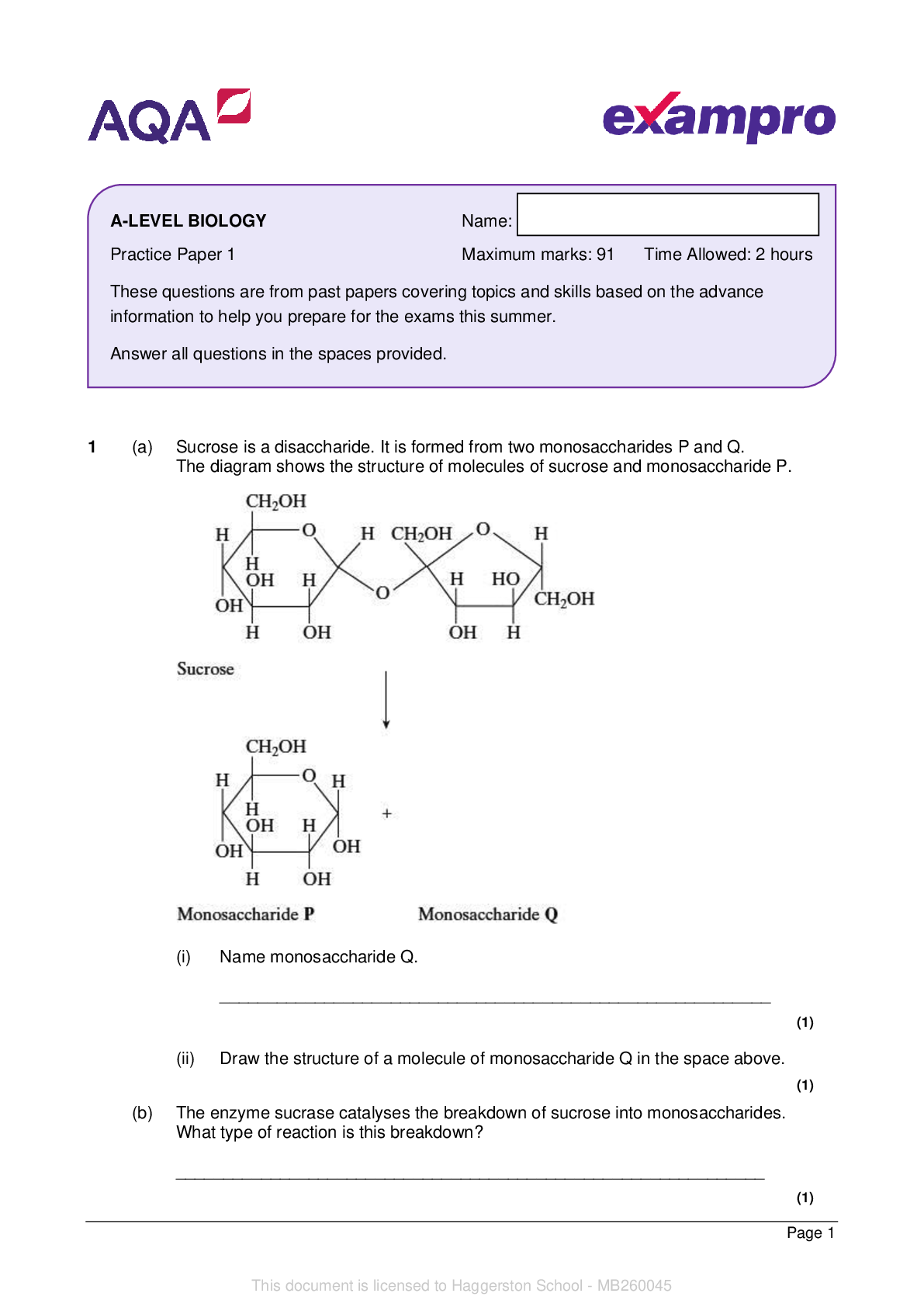
1 Within the permafrost in Arctic regions of the Earth, large amounts of methane are trapped within ice as ‘methane hydrate’, CH4•xH2O. Methane makes up about 13.4% of the mass of ‘methane h... ydrate’. Scientists are concerned that global warming will melt the permafrost, releasing large quantities of methane into the atmosphere. (a) The H–O–H bond angle in ice is about 109° but about 105° in gaseous H2O. Explain why there is this difference. ................................................................................................................................................... ................................................................................................................................................... ................................................................................................................................................... ................................................................................................................................................... ................................................................................................................................................... ............................................................................................................................................. [3] (b) Why are scientists concerned about the release of methane into the atmosphere? ................................................................................................................................................... ................................................................................................................................................... ............................................................................................................................................. [1] (c) Determine the formula of ‘methane hydrate’, CH4•xH2O. In the formula, show the value of x to two decimal places. formula = ......................................................... [2] PMT3 © OCR 2017 Turn over (d) Calculate the volume of methane, in dm3, that would be released from the melting of each 1.00 kg of ‘methane hydrate’ at 101 kPa and 0 °C. Give your answer to three significant figures. volume = ................................................. dm3 [4] (e) Suggest why some industries are interested in the presence of ‘methane hydrate’ in regions of the Earth. ................................................................................................................................................... ................................................................................................................................................... ............................................................................................................................................. [1] PMT4 © OCR 2017 2 A student plans to determine the enthalpy change of reaction 3.1 shown below. Na 2O(s) + 2HCl(aq) 2NaCl(aq) + H2O(l) reaction 3.1 This enthalpy change can be determined indirectly using Hess’ Law from the enthalpy changes of reaction 3.2 and reaction 3.3 shown below. Na 2O(s) + H2O(l) 2NaOH(aq) reaction 3.2 HCl(aq) + NaOH(aq) NaCl(aq) + H2O(l) ∆rH = −57.6 kJ mol−1 reaction 3.3 The student will determine the enthalpy change of reaction 3.2 as outlined below. • Weigh a bottle containing Na2O(s) and weigh a polystyrene cup. • Add about 25 cm3 of water to the polystyrene cup and measure its temperature. • Add the Na 2O(s), stir the mixture, and measure the maximum temperature reached. • Weigh the empty bottle and weigh the polystyrene cup with the final solution. Mass readings Mass of bottle + Na 2O(s) = 16.58 g Mass of empty bottle = 15.34 g Mass of empty polystyrene cup = 21.58 g Mass of polystyrene cup + final solution = 47.33 g Temperature readings Initial temperature of water = 20.5 °C Maximum temperature of final solution = 55.5 °C The density and specific heat capacity, c, of the solution are the same as for water. [Show More]
Last updated: 1 year ago
Preview 1 out of 20 pages

Reviews( 0 )
Document information
Connected school, study & course
About the document
Uploaded On
Jun 20, 2021
Number of pages
20
Written in
Additional information
This document has been written for:
Uploaded
Jun 20, 2021
Downloads
0
Views
813