Chemistry > QUESTIONS & ANSWERS > CHM 115 Chapter 19 & Thermodynamics 20 Practice Questions with answers (All)
CHM 115 Chapter 19 & Thermodynamics 20 Practice Questions with answers
Document Content and Description Below
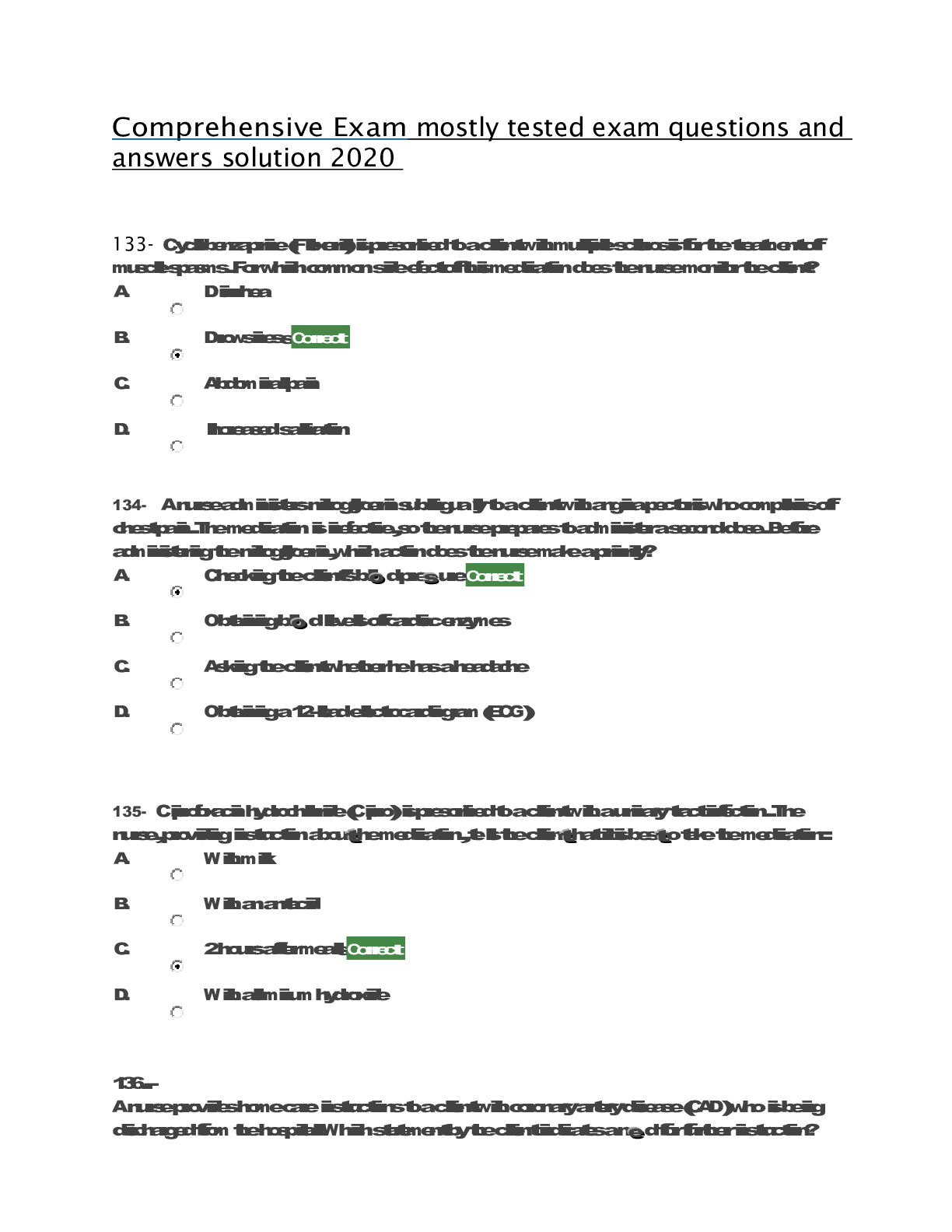
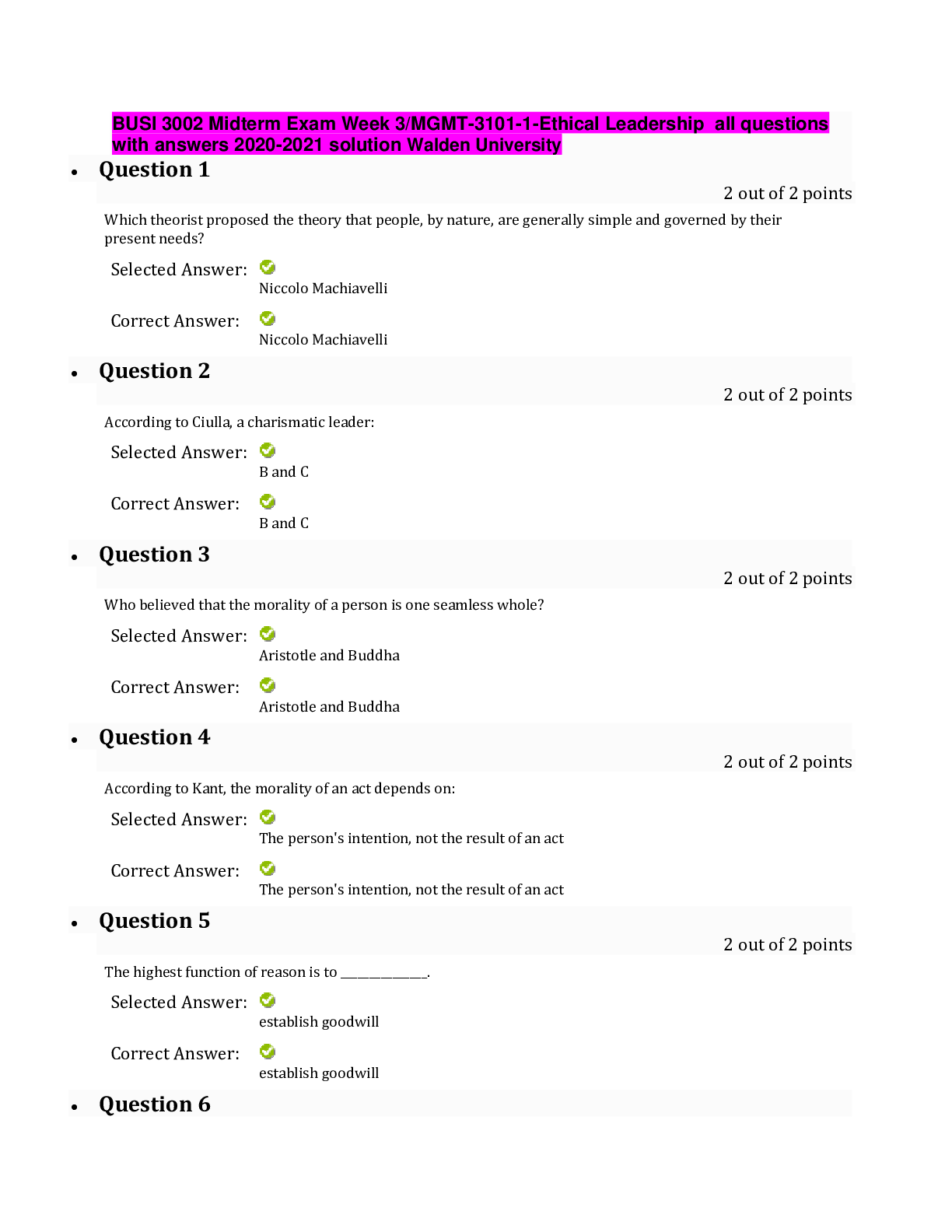
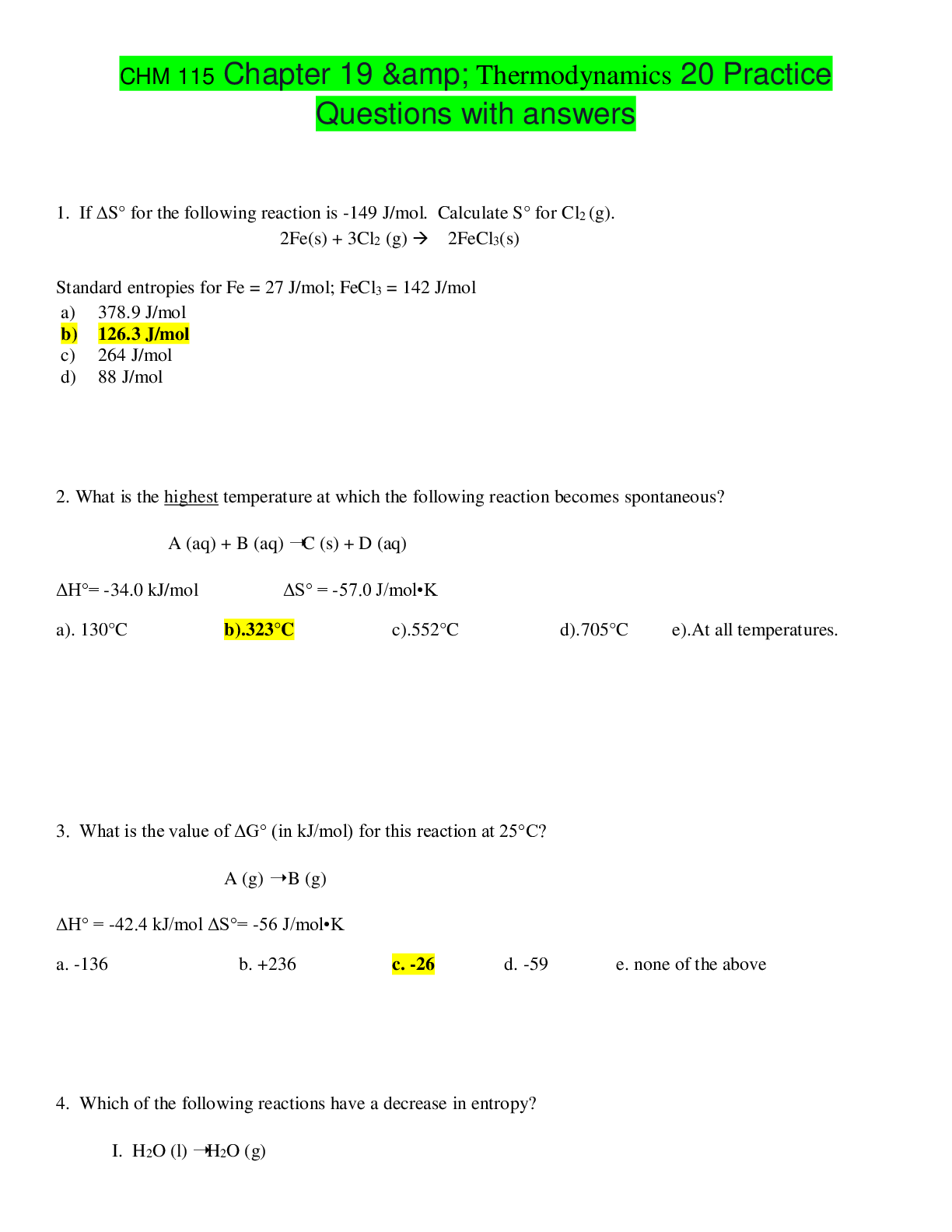
CHM 115 Chapter 19 & Thermodynamics 20 Practice Questions with answers 1. If S° for the following reaction is -149 J/mol. Calculate S° for Cl2 (g). 2Fe(s) + 3Cl2 (g) 2FeCl3(s)... Standard entropies for Fe = 27 J/mol; FeCl3 = 142 J/mol 2. What is the highest temperature at which the following reaction becomes spontaneous? A (aq) + B (aq) ➝C (s) + D (aq) ΔH°= -34.0 kJ/mol ΔS° = -57.0 J/mol•K 3. What is the value of ΔG° (in kJ/mol) for this reaction at 25°C? A (g) ➝ B (g) ΔH° = -42.4 kJ/mol ΔS°= -56 J/mol•K 4. Which of the following reactions have a decrease in entropy? I. H2O (l) ➝H2O (g) II. 2 H2 (g) + O2 (g) ➝ 2 H2O (g) III. MgO (s) + CO2 (g) ➝ MgCO3 (s) IV. PCl5 (s) ➝ PCl3 (l) + Cl2 (g) 5. Calculate the entropy change (J/mole.K) of the reaction. The entropies [S in J/mol K] are given in brackets after each substance. 2C2H2 (g) [200.8] + 5 O2 (g) [205.0] ➝ 4CO2 (g) [213.6] + 2H2O (l) [69.91] 6. Which statement is true if the following reaction is done at 25 oC? H2 (g) + I2(g) 2HI(g) ∆Hfo, kJ /mol 0 0 26.5 ∆Sfo, J /mol•K 130.6 260.6 206.5 7. Which of the following reactions would have a positive value for ΔS°? I. NH3(g) + HCl(g) NH4Cl(s) II. Ag+(aq) + Cl-(aq) AgCl(s) III. 2H2(g) + O2(g) 2H2O(g) IV. C6H10Br2 (l) C6H10 (l) + Br2 (l) (more moles on products) V. 2H2O2(g) 2H2O(g) + O2(g) (more moles on products) 8. Calculate ΔG0 (kJ/mol) for the reaction using the listed [ΔG0f values]: P2 (g) [103.7] + O2 (g)[0] + 3Cl2 (g) [0]➝ 2POCl3 (g) [-502.5] 9. Liquid water freezes spontaneously at -5oC and 1 atm.Which of the following statements is most likely TRUE? a). Liquid water freezes at -5oC because the increase in entropy overcomes the endothermicity. b). Liquid water freezes at -5oC because the decrease in entropy overcomes the endothermicity. c). Liquid water freezes at -5oC because the exothermicity overcomes the decrease in entropy. d). Liquid water freezes at -5oC because the exothermicity overcomes the increase in entropy. e). Liquid water freezes at -5oC because the endothermicity overcomes the decrease in entropy. 10. Hydrogen peroxide decomposes according to the following reaction: H2O2 (l) H2O(l) + ½ O2(g) For this reaction ∆H0 = -98.2 kJ and ∆S0 = 70.1 J/K. Which is the approximate value of Kp for this reaction at 25oC? 11. Which one of the following cannot be determined using thermodynamics? a). the direction of a spontaneous reaction b). the temperature at which a reaction will be spontaneous c). the extent of a reaction d). the rate of a reaction e). the value of the equilibrium constant. 12. A reaction that is spontaneous as written __________. a). will proceed without outside intervention. b). has an equilibrium position that lies far to the left. c). is also spontaneous in the reverse direction. d). is very rapid. e). is very slow. 13. Which of the following reactions would have aincrease value for ΔS°? I. H2O (l) ➝ H2O (g) (more moles of gas on product) II. SCl5 (s) ➝ SCl3 (l) + Cl2 (g)(more moles of gas on product) III. 2 H2 (g) + O2 (g) ➝ 2 H2O (g) IV. CaO (s) + CO2 (g) ➝CaCO3 (s) 14. The reaction rates for many spontaneous reactions are actually very slow. Which of the following is the best explanation for this observation? a). Kp for the reaction = 0. b). The activation energy is high. c). The standard free energy change for the reaction is > 0. d). These reactions are endothermic. e). The standard entropy change is < 0. 15. The following reaction has a ΔG° value of 32.64 kJ/mol at 25°C. HB(aq) + H2O(l) H3O+(aq) + B–(aq) Calculate the equilibrium constant, Ka, for the acid HB. a) 0.987 b) –13.2 c) 1.90 × 10–6 d) 3.26 × 10–5 e) –157 16. For which process is S negative? a) evaporation of 1 mol of CCl4(l) b) mixing 5 mL ethanol with 25 mL water c) compressing 1 mol Ne at constant temperature from 1.5 L to 0.5 L d) raising the temperature of 100 g Cu from 275 K to 295 K e) grinding a large crystal of KCl to powder 17. For a particular chemical reaction H = 6.8 kJ and S = –29 J/K. Under what temperature condition is the reaction spontaneous? a) When T < –234 K. b) When T < 234 K. c) The reaction is spontaneous at all temperatures. d) The reaction is not spontaneous at any temperature. e) When T> 234 K. 18. For the reaction A + B C + D, H° = +40 kJ and S° = +50 J/K. Therefore, the reaction under standard conditions is a) spontaneous at temperatures less than 10 K b) spontaneous at temperatures greater than 800 K c) spontaneous only at temperatures between 10 K and 800 K d) spontaneous at all temperatures e) nonspontaneous at all temperatures 19. Consider the following hypothetical reaction at 310 K. Standard free energies of formation are given in parentheses. B C G° = –46.0 kJ/mol (?) (176.4 kJ/mol) Calculate the standard free energy of formation of compound B. a) 222.4 kJ/mol b) –222.4 kJ/mol c) 130.4 kJ/mol d) –130.4 kJ/mol e) none of these 20. Water gas, a commercial fuel, is made by the reaction of hot coke carbon with steam. C(s) + H2O(g) CO(g) + H2(g) When equilibrium is established at 828°C the concentrations of CO, H2, and H2O are 4.00 10–2, 4.00 10–2, and 1.00 10–2 mole/liter, respectively. Calculate the value of G° for this reaction at 828°C. a) 12.6 kJ b) –12.69 kJ c) 54.84 kJ d) 16.77 kJ e) none of these Electrochemistry 21. What is the oxidation state of Hg in Hg2Cl2? 22. Which of the following is true for the cell shown here? Zn(s)│Zn2+(aq) ║ Cr3+(aq) │Cr(s) a) The electrons flow from the cathode to the anode. b) The electrons flow from the zinc to the chromium. c) The electrons flow from the chromium to the zinc. d) The chromium is oxidized. e) The zinc is reduced. 23. What is the balanced chemical equation corresponding to the following cell diagram? K(s) | K+(aq) || Sn2+(aq) | Sn(s) a) 2K(s) + 2K+(aq) → Sn2+(aq) + Sn(s) b) Sn(s) + 2K+(aq) → 2K(s) + Sn2+(aq) c) 2K(s) + Sn(s) → 2K+(aq) + Sn2+(aq) d) Sn2+(aq) + Sn(s) → 2K(s) + 2K+(aq) e) 2K(s) + Sn2+(aq) → 2K+(aq) + Sn(s) 24. What is ℰ°cell for the following electrochemical equation? 2Ag(s) + Ti2+(aq) → 2Ag+(aq) + Ti(s) (ℰ°red(Ag+/Ag) = 0.8 V, ℰ°red(Ti2+/Ti) = –1.63 V) a) –0.030 V b) –3.230 V c) –2.430 V d) 2.430 V e) 3.230 V 25. The galvanic cell described by Zn(s)│Zn2+(aq)║Cu2+(aq)│Cu(s) has a standard cell potential of 1.101 volts. Given that Zn(s) Zn2+(aq) + 2e– has an oxidation potential of 0.762 volts, determine the reduction potential for . a) 1.863 V b) –1.863 V c) –0.339 V d) 0.339 V e) none of these 26. For a certain reaction, H° = –77.6 kJ and S° = –217 J/K. If n = 3, calculate ℰ° for the reaction at 25°C. a) 0.0447 V b) 0.491 V c) 0.287 V d) 0.134 V e) 0.249 V 27. How many seconds would it take to deposit 17.3 g of Ag (atomic mass = 107.87) from a solution of AgNO3 using a current of 10.00 amp? a) 3.09 103 s b) 7.74 102 s c) 4.64 103 s d) 1.55 103 s e) 5.16 102 s 28. Given the following reaction: Cr2O7-2 + S2O32- + H+ Cr3+ + S4O62- + H2O What is the reducing agent? a. Cr2O7-2 b. S2O32- c. Cr3+ d. S4O62- e. H + 29. What are the oxidation states of sulfur and fluorine, respectively, in the molecule, SF6? a. +1 : -1 b. +1 : +1 c. +3 : -1 d. +6 : -1 e. -6 : +1 30. How many seconds will be required to produce 1.0 g of chromium metal by the electrolysis of a Cr(NO3)3 solution using a current of 3.0 A? a. 3.6 x 101 b. 6.3 x 102 c. 9.7 x 102 d. 1.9 x 103 e. 3.7 x 103 Consider the following standard reduction potentials for the next two questions: half reaction Eo, V Ca2+ + 2e- --> Ca -2.76 (RED) Pb2+ + 2e- --> Pb -0.13 Cu2+ + 2e- --> Cu +0.34 Hg22+ + 2e- -->2Hg +0.80 Pt2+ + 2e- --> Pt +1.20 (OX) 31. Which of the following metals is the strongest REDUCING AGENT? a. Ca b. Pb c. Cu d. Hg e. Pt 32. Which of the following metals is the strongest OXIDIZING AGENT? a. Ca2+ b. Pb2+ c. Cu2+ d. Hg22+ e. Pt2+ 33.How many electrons are transferred in the following reaction? Cd + 2HCl CdCl2 + H2 a) 0 b) 1 c) 2 d) 4 e) not enough information given 34. Which of the following statements is true concerning the electrochemical cell depicted below? Ca | Ca2+(aq) || Mn2+(aq) | Mn Ca2+(aq) + 2e– Ca(s); ℰ° = –2.87 V Mn2+(aq) + 2e– Mn(s); ℰ° = –1.19 V a) The cell reaction is spontaneous with a standard cell potential of 1.68 V. b) The cell reaction is spontaneous with a standard cell potential of 4.06 V. c) The cell reaction is nonspontaneous with a standard cell potential of –1.68 V. d) The cell reaction is nonspontaneous with a standard cell potential of –4.06 V. e) The cell is at equilibrium. 35. The reduction potentials for Ni2+ and Sn2+ are as follows: Ni2+ + 2e– Ni, ℰ° = –0.231 V Sn2+ + 2e– Sn, ℰ° = –0.140 V Calculate the equilibrium constant at 25 °C for the reaction: Sn2+ + Ni Sn + Ni2+ a) 3.6 1012 b) 35 c) 5.9 d) 8.3 10–4 e) 1.2 103 36. What is ℰ of the following cell reaction at 25°C? ℰ°cell = 0.460 V. Cu(s) | Cu2+(0.017 M) || Ag+(0.17 M) | Ag(s) a) 0.282 V b) 0.476 V c) 0.460 V d) 0.467 V e) 0.490 V 37. When the following reaction is balanced in ACIDIC solution, what is the coefficient for S2O32-? Cr2O7-2 + S2O32- Cr3+ + S4O62- a. 3 b. 4 c. 5 d. 6 e. 7 38. When the following reaction is balanced in BASIC solution, what is the coefficient for S? S2- + MnO4- S + MnO2 a. 1 b. 2 c. 3 d. 4 e. 5 39. Which reaction correctly describes the electro-chemical cell shown? a. 2Al3+ + 3Pb+2 2Al + 3Pb b. 2Al3+ + 3Pb 3Pb2+ + 2Al c. 2Al + 3Pb+2 2Al3+ + 3Pb d. 2Al + 3Pb 2Al3+ + 3Pb+2 40. What voltage will be produced by the electrochemical cell shown above? Al3+ + 3e– Al, ℰ° = –1.66 V Pb2+ + 2e– Pb, ℰ° = –0.13 V a. 1.79 V b 1.53 V c. -1.53 V d. -1.79 V e.3.06 V 41. The following half-reactions indicate the reduction potentials for an electrochemical cell: half reaction Eo, V Cu+ + e-Cu 0.52 Fe3+ + e-Fe2+ 0.77 At standard conditions, what species are produced at each electrode? a. Cu is produced at the cathode and Fe2+ at the anode. b. Cu is produced at the cathode and Fe3+ at the anode. c. Fe is produced at the cathode and Cu+ at the anode. d. Fe3+ is produced at the cathode and Cu+ at the anode. e. Fe2+ is produced at the cathode and Cu+ is produced at the anode. 42. A product–favored electrochemical reaction has: a. ΔG° = 0, E° = 0, and K >> 1 b. ΔG° < 0, E° > 0, and K > 1 c. ΔG° > 0, E° < 0, and K < 1 d. ΔG° > 0, E° < 0, and K > 1 e. ΔG° < 0, E° = 0, and K >> 1 43. Consider an electrochemical cell based on the following overall reaction, Fe(s) + 2Ag+(aq) Fe2+(aq) + 2Ag(s) Fe2+(aq) + 2e– Fe(s), ℰ° = –0.44 V Ag+(aq) + e– Ag, ℰ° = 0.80 V Calculate the cell potential (in V) for this reaction at 25oC when the concentration of Ag+ ions is 0.050 M and the concentration of Fe2+ ions is 1.50 M. a. +1.32 V b. +1.50 V c. +1.20 V d. -1.32 V e. -1.20 V 44. ________________ is the loss of electrons from an ion or compound and ________________ is the addition of electrons to an ion or compound. During an oxidation-reduction reaction, electrons are transferred from the ________________¬¬¬¬¬¬______ to the _________________________. a. oxidation; reduction; reducing agent; oxidizing agent b. oxidation; reduction; oxidizing agent; reducing agent c. reduction; oxidation; reducing agent; oxidizing agent d. reduction; oxidation; oxidizing agent; reducing agent 45. Which reaction correctly describes the electro-chemical cell shown? a. Cu2+(aq) + 2Ag+ (aq) Cu (s) + 2Ag (s) b. Cu(s) + 2Ag (s) Cu2+(aq) + 2Ag+ (aq) c. Cu2+(aq) + 2Ag (s) Cu (s) + 2Ag+ (aq) d. Cu(s) + 2Ag+ (aq) Cu2+ (aq) + 2Ag (s) 46. What voltage will be produced by the electrochemical cell described in the problem above? Cu2+ + 2e– Cu, ℰ° = 0.34 V Ag+ + e– Ag, ℰ° = 0.80 V a. +1.14 V b. +0.46 V c. +1.48 V d. -1.14 V e. -0.46 V 47. Which description of the cell in question 45 is correct? a. e’s flow from the Cu cathode to the Ag anode and K+ ions diffuse into the AgNO3 solution. b. e’s flow from the Cu cathode to the Ag anode and K+ ions diffuse into the Cu(NO3)2 solution. c. e’s flow from the Cu anode to the Ag cathode and K+ ions diffuse into the Cu(NO3)2 solution. d. e’s flow from the Cu anode to the Ag cathode and K+ ions diffuse into the AgNO3 solution. 48. Calculate Ecell for the following voltaic cell. Ag / Ag+(1.0 × 10-5M) // Au3+(1.0 × 10-1M) / Au Au3+ + 3e– Au, ℰ° = 1.50 V Ag+ + e– Ag, ℰ° = 0.80 V a. +0.78 V b. +0.46 V c. +0.98 V d. +2.58 V e. -0.78 V 49. What is the correct shorthand notation for the voltaic cell that utilizes the reaction below. Fe(s) + Cu2+(aq) Fe2+(aq) + Cu(s) fa. Fe(s) / Fe2+(aq) // Cu2+(aq) / Cu(s) b. Cu(s)/Cu2+(aq)//Fe2+(aq)/Fe(s) c. Cu2+(aq) / Cu(s)//Fe2+(aq)/Fe(s) d. Fe2+(aq)/Fe(s)//Cu2+(aq) / Cu(s) e. Fe2+(aq)/Fe(s)//Cu(s)/Cu2+(aq) 50. Solutions of Ag+, Cu2+, Fe3+ and Ti4+ are electrolyzed with a constant current until 0.10 mol of metal is deposited. Which will require the greatest length of time? a. Ag+ b. Cu2+ c. Ti4+ d. Fe3+ e. The length of time is the same for all ions [Show More]
Last updated: 1 year ago
Preview 1 out of 14 pages
Buy this document to get the full access instantly
Instant Download Access after purchase
Add to cartInstant download
We Accept:

Reviews( 0 )
$11.00
Document information
Connected school, study & course
About the document
Uploaded On
Oct 23, 2020
Number of pages
14
Written in
Additional information
This document has been written for:
Uploaded
Oct 23, 2020
Downloads
0
Views
68