Chemistry > QUESTIONS & ANSWERS > Questions and Answers > CH 102Lab 11 PostLab - Redox Reactions Current Score : 22 / 25 (All)
Questions and Answers > CH 102Lab 11 PostLab - Redox Reactions Current Score : 22 / 25
Document Content and Description Below
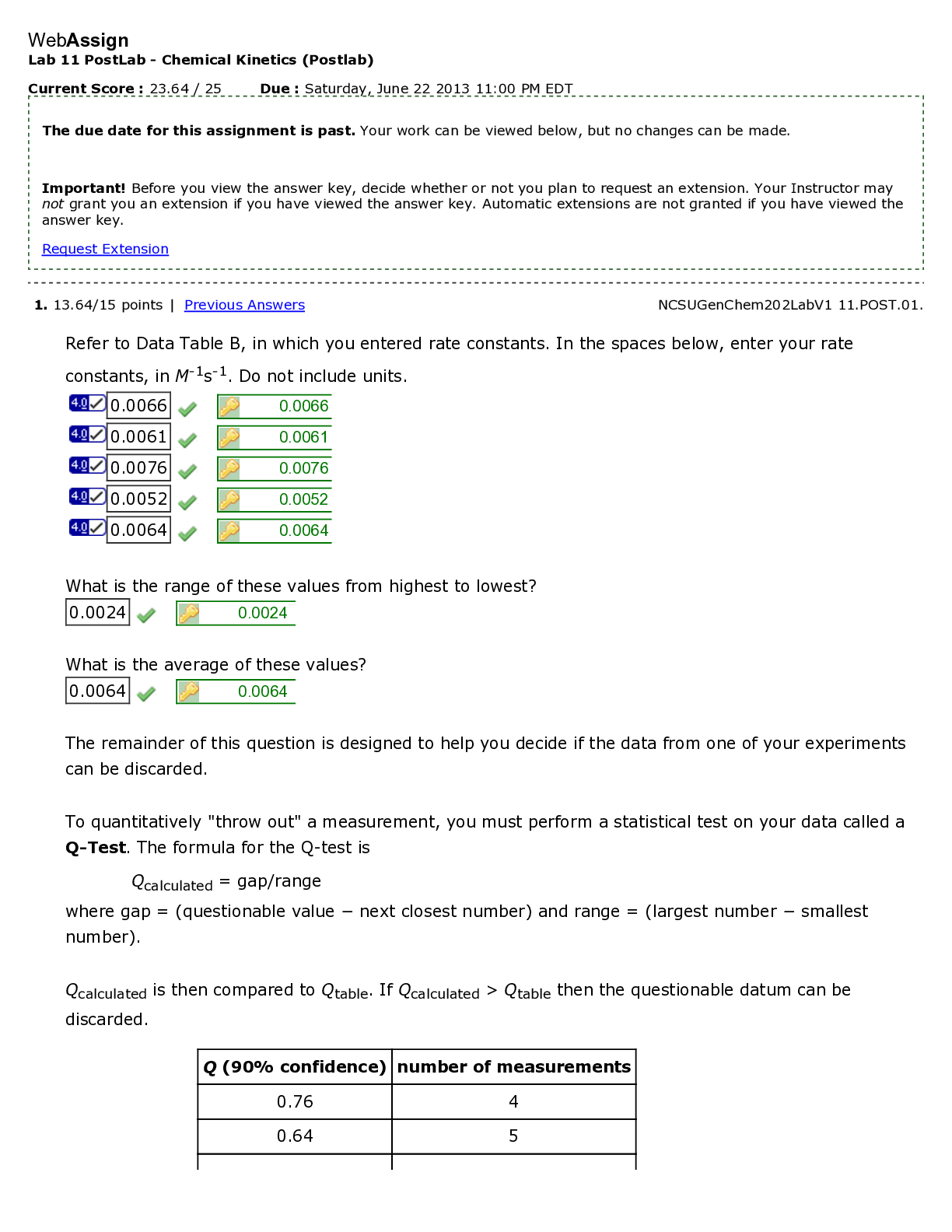
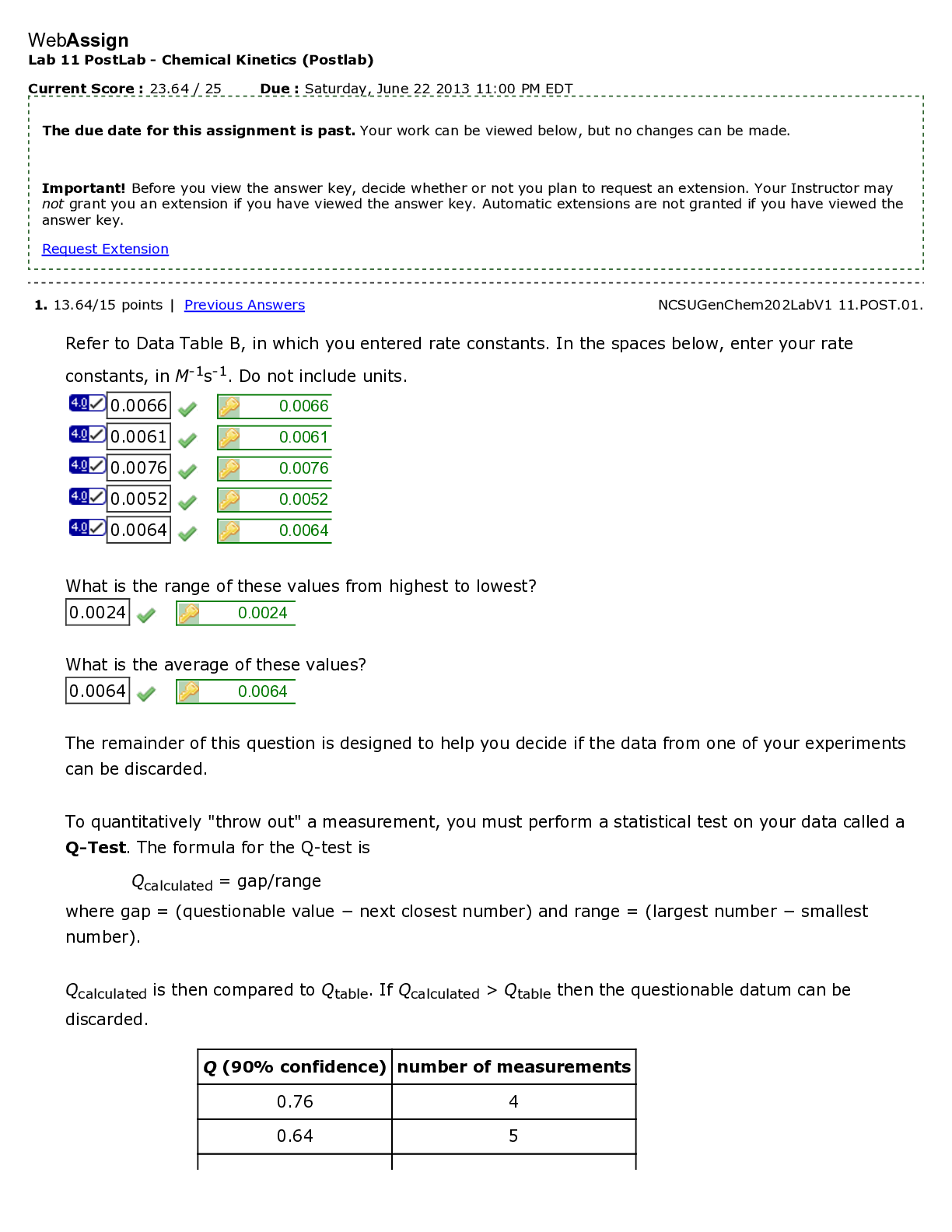
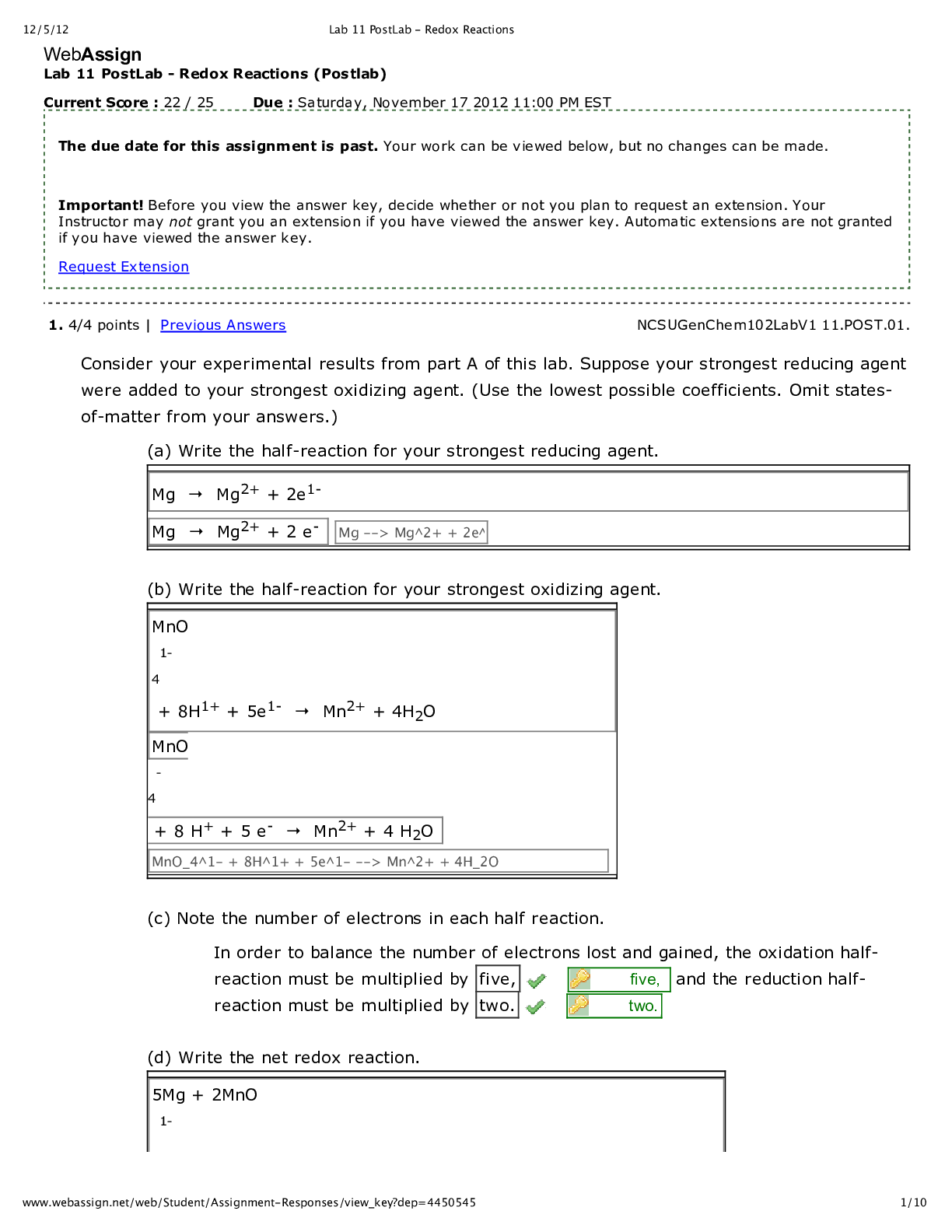
WebAssign Lab 11 PostLab - Redox Reactions (Postlab) Current Score : 22 / 25 Due : Saturday, November 17 2012 11:00 PM EST 1. 4/4 points | Previous Answers NCSUGenChem102LabV1 11.POST.01. ... Consider your experimental results from part A of this lab. Suppose your strongest reducing agent were added to your strongest oxidizing agent. (Use the lowest possible coefficients. Omit states- of-matter from your answers.) (a) Write the half-reaction for your strongest reducing agent. (b) Write the half-reaction for your strongest oxidizing agent. (c) Note the number of electrons in each half reaction. In order to balance the number of electrons lost and gained, the oxidation half- reaction must be multiplied by five, five, and the reduction half- reaction must be multiplied by two. two. (d) Write the net redox reaction. Redox Reactions 2. 1/3 points | Previous Answers NCSUGenChem102LabV1 11.POST.02. Students should always be careful of the chemical reaction between jewelry and laboratory reagents. The Standard Reduction Potentials Table shows the reduction reactions and potentials for some common lab reagents and metals. (a) What is the net redox reaction that occurs when Au comes into contact with acidic KMnO4? (Use the lowest possible coefficients. Omit states-of-matter from your answer.) (b) What is E°cell for the reaction? (c) Which metal is the most reactive? (d) Which reagent is the most reactive? Redox Reactions 3. 4/4 points | Previous Answers NCSUGenChem102LabV1 11.POST.03. During part B of your lab, you measured electrochemical cell potentials with a Cu2+/Cu couple as the anode. Predict the results you would observe if Zn2+/Zn were the anode. Redox Reactions 4. 4/4 points | Previous Answers NCSUGenChem102LabV1 11.POST.04. In part B of Lab 11, you measured the voltage of several galvanic cells. Assemble a battery, represented by the diagram below with the cathode in compartment A, with Zn2+/Zn and Fe2+/Fe couples in which the voltage reads positive. (Use the Standard Reduction Potentials Table. Use the lowest possible coefficients. Omit states-of-matter from your answer.) (a) What half-reaction occurs in compartment A? (b) What half-reaction occurs in compartment B? (c) Write the net redox reaction. (d) What is the cell potential? .32 V Redox Reactions 5. 4/5 points | Previous Answers NCSUGenChem102LabV1 11.POST.05. Green checks and red X's are not displayed for this question. Determine the errors (if any) with each galvanic cell set-up when the anode is on the left. (Select all that apply.) Redox Reactions 6. 4.5/4.5 points | Previous Answers NCSUGenChem102LabV1 11.POST.06. In Lab 9, students performed acid-base titrations. Redox reactions can also be used in titrations. An example is the titration of ascorbic acid (H2C6H6O6) in lemon juice using triiodide (I3– ). A starch indicator will turn the solution blue-black at the endpoint. The half-reactions involved are shown below. (a) What is the net redox reaction that occurs? (Use the lowest possible coefficients. Omit states-of-matter from your answer.) (b) What is the stoichiometry of H2C6H6O6 to I3–? (c) Use the data given below to determine the amount of ascorbic acid in lemon juice. (Note: The recommended daily allowance of ascorbic acid (Vitamin C) is 90 mg.) Data Table P6: Titration of ascorbic acid in lemon juice with triiodide Redox Reactions 7. 0.5/0.5 points | Previous Answers NCSUGenChem102LabV1 11.POST.07. You are now answering the last question of the last Postlab assignment in CH102. Which of the following words or phrases best describes your emotions at this point? [Show More]
Last updated: 1 year ago
Preview 1 out of 10 pages
Instant download
Buy this document to get the full access instantly
Instant Download Access after purchase
Add to cartInstant download
Reviews( 0 )
Document information
Connected school, study & course
About the document
Uploaded On
Nov 11, 2020
Number of pages
10
Written in
Additional information
This document has been written for:
Uploaded
Nov 11, 2020
Downloads
1
Views
223