*NURSING > QUESTIONS & ANSWERS > NR565 Week 4 Study Guide Chapter 8. An Introduction to Pharmacogenomics. Questions and Answers list (All)
NR565 Week 4 Study Guide Chapter 8. An Introduction to Pharmacogenomics. Questions and Answers listed at the end. Best for the quick, near exam study.
Document Content and Description Below
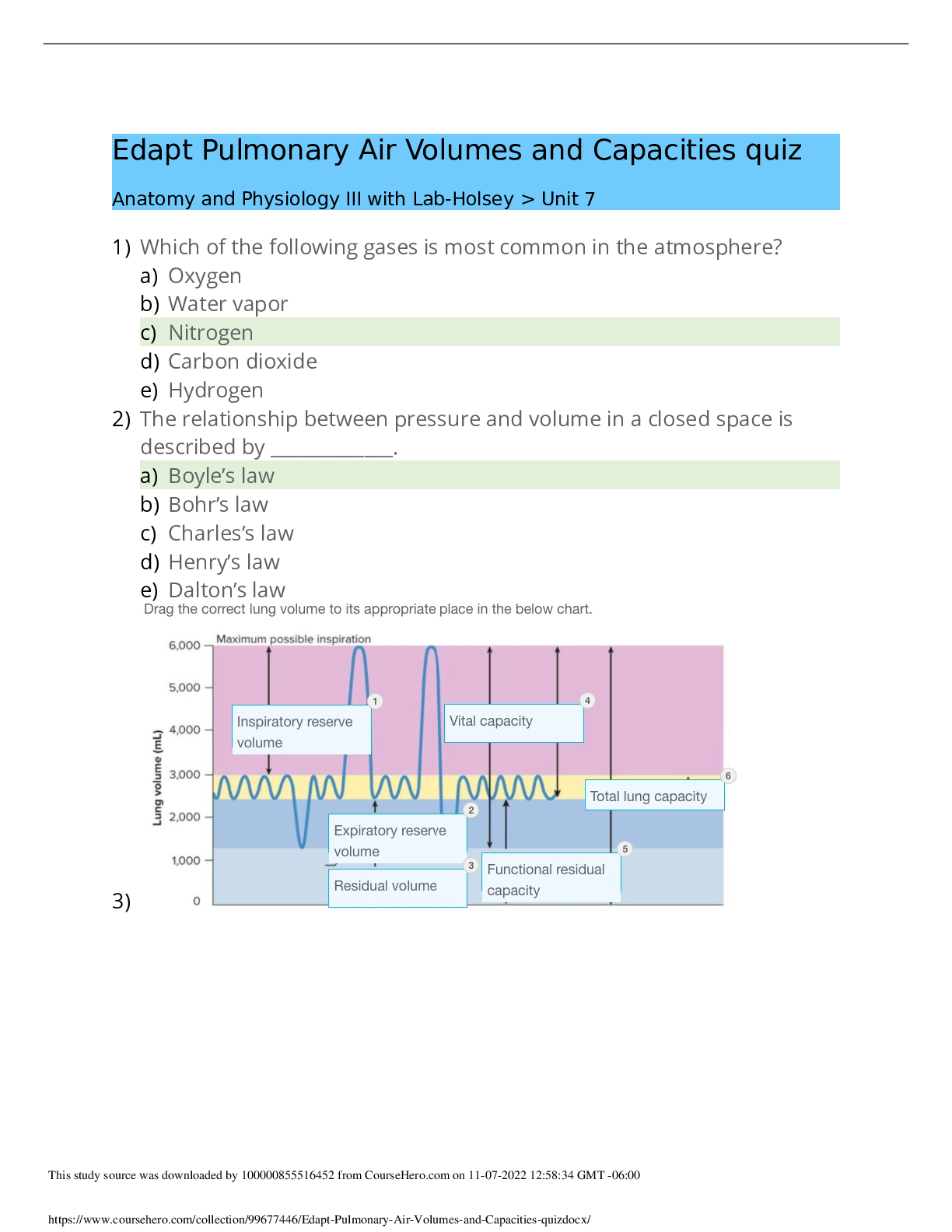
NR565 Week 4 Study Guide Chapter 8. An Introduction to Pharmacogenomics Multiple Choice Identify the choice that best completes the statement or answers the question. ____ 1. Genetic polymorp... hisms account for differences in metabolism, including: 1. Poor metabolizers, who lack a working enzyme 2. Intermediate metabolizers, who have one working, wild-type allele and one mu-tant allele 3. Extensive metabolizers, with two normally functioning alleles 4. All of the above ____ 2. Up to 21% of Asians are ultra-rapid 2D6 metabolizers, leading to: 1. A need to monitor drugs metabolized by 2D6 for toxicity 2. Increased dosages needed of drugs metabolized by 2D6, such as the selective ser-otonin reuptake inhibitors 3. Decreased conversion of codeine to morphine by CYP 2D6 4. The need for lowered dosages of drugs, such as beta blockers ____ 3. Rifampin is a nonspecific CYP450 inducer that may: 1. Lead to toxic levels of rifampin and must be monitored closely 2. Cause toxic levels of drugs, such as oral contraceptives, when coadministered 3. Induce the metabolism of drugs, such as oral contraceptives, leading to therapeu-tic failure 4. Cause nonspecific changes in drug metabolism ____ 4. Inhibition of P-glycoprotein by a drug such as quinidine may lead to: 1. Decreased therapeutic levels of quinidine 2. Increased therapeutic levels of quinidine 3. Decreased levels of a coadministered drug, such as digoxin, that requires P-glycoprotein for absorption and elimination 4. Increased levels of a co-administered drug, such as digoxin, that requires P-glycoprotein for absorption and elimination ____ 5. Warfarin resistance may be seen in patients with VCORC1 mutation, leading to: 1. Toxic levels of warfarin building up 2. Decreased response to warfarin 3. Increased risk for significant drug interactions with warfarin 4. Less risk of drug interactions with warfarin ____ 6. Genetic testing for VCORC1 mutation to assess potential warfarin resistance is required prior to prescribing warfarin. 1. True 2. False ____ 7. Pharmacogenetic testing is required by the U.S. Food and Drug Administration prior to prescribing: 1. Erythromycin 2. Digoxin 3. Cetuximab 4. Rifampin ____ 8. Carbamazepine has a Black Box Warning recommending testing for the HLA-B*1502 allele in patients with Asian ancestry prior to starting therapy due to: 1. Decreased effectiveness of carbamazepine in treating seizures in Asian patients with the HLA-B*1502 allele 2. Increased risk for drug interactions in Asian patients with the HLA-B*1502 allele 3. Increased risk for Stevens-Johnson syndrome in Asian patients with HLA-B*1502 allele 4. Patients who have the HLA-B*1502 allele being more likely to have a resistance to carbamazepine ____ 9. A genetic variation in how the metabolite of the cancer drug irinotecan SN-38 is inactivated by the body may lead to: 1. Decreased effectiveness of irinotecan in the treatment of cancer 2. Increased adverse drug reactions, such as neutropenia 3. Delayed metabolism of the prodrug irinotecan into the active metabolite SN-38 4. Increased concerns for irinotecan being carcinogenic ____ 10. Patients who have a poor metabolism phenotype will have: 1. Slowed metabolism of a prodrug into an active drug, leading to accumulation of prodrug 2. Accumulation of inactive metabolites of drugs 3. A need for increased dosages of medications 4. Increased elimination of an active drug ____ 11. Ultra-rapid metabolizers of drugs may have: 1. To have dosages of drugs adjusted downward to prevent drug accumulation 2. Active drug rapidly metabolized into inactive metabolites, leading to potential therapeutic failure 3. Increased elimination of active, nonmetabolized drug 4. Slowed metabolism of a prodrug into an active drug, leading to an accumulation of prodrug ____ 12. A provider may consider testing for CYP2D6 variants prior to starting tamoxifen for breast cancer to: 1. Ensure the patient will not have increased adverse drug reactions to the tamoxifen 2. Identify potential drug-drug interactions that may occur with tamoxifen 3. Reduce the likelihood of therapeutic failure with tamoxifen treatment 4. Identify poor metabolizers of tamoxifen Chapter 8. An Introduction to Pharmacogenomics Answer Section [Show More]
Last updated: 1 year ago
Preview 1 out of pages

Buy this document to get the full access instantly
Instant Download Access after purchase
Add to cartInstant download
We Accept:

Reviews( 0 )
$8.00
Document information
Connected school, study & course
About the document
Uploaded On
Nov 19, 2020
Number of pages
Written in
Additional information
This document has been written for:
Uploaded
Nov 19, 2020
Downloads
0
Views
6