BioChemistry > STUDY GUIDE > Biochem C785 Kaleys Comprehensive Study Guide final (All)
Biochem C785 Kaleys Comprehensive Study Guide final
Document Content and Description Below
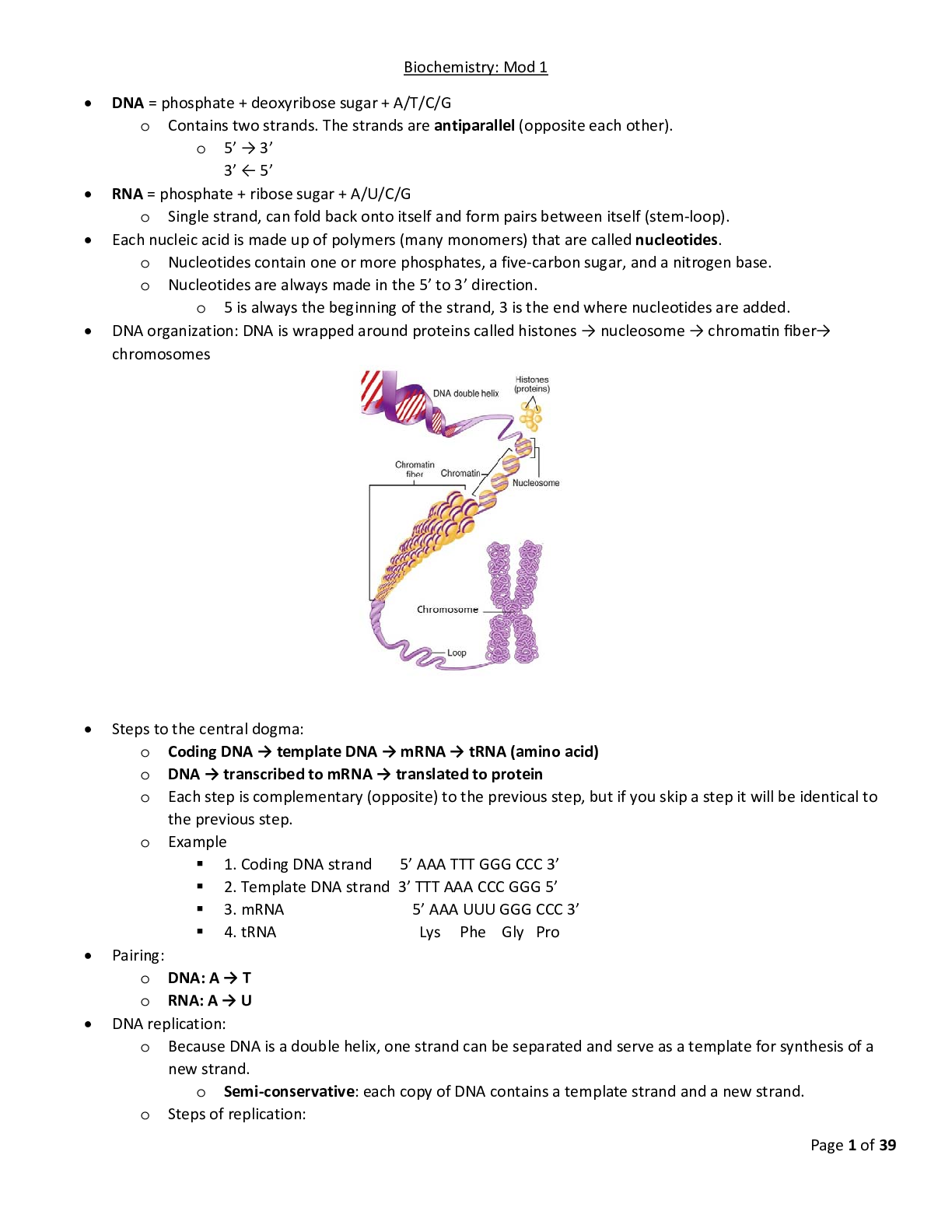
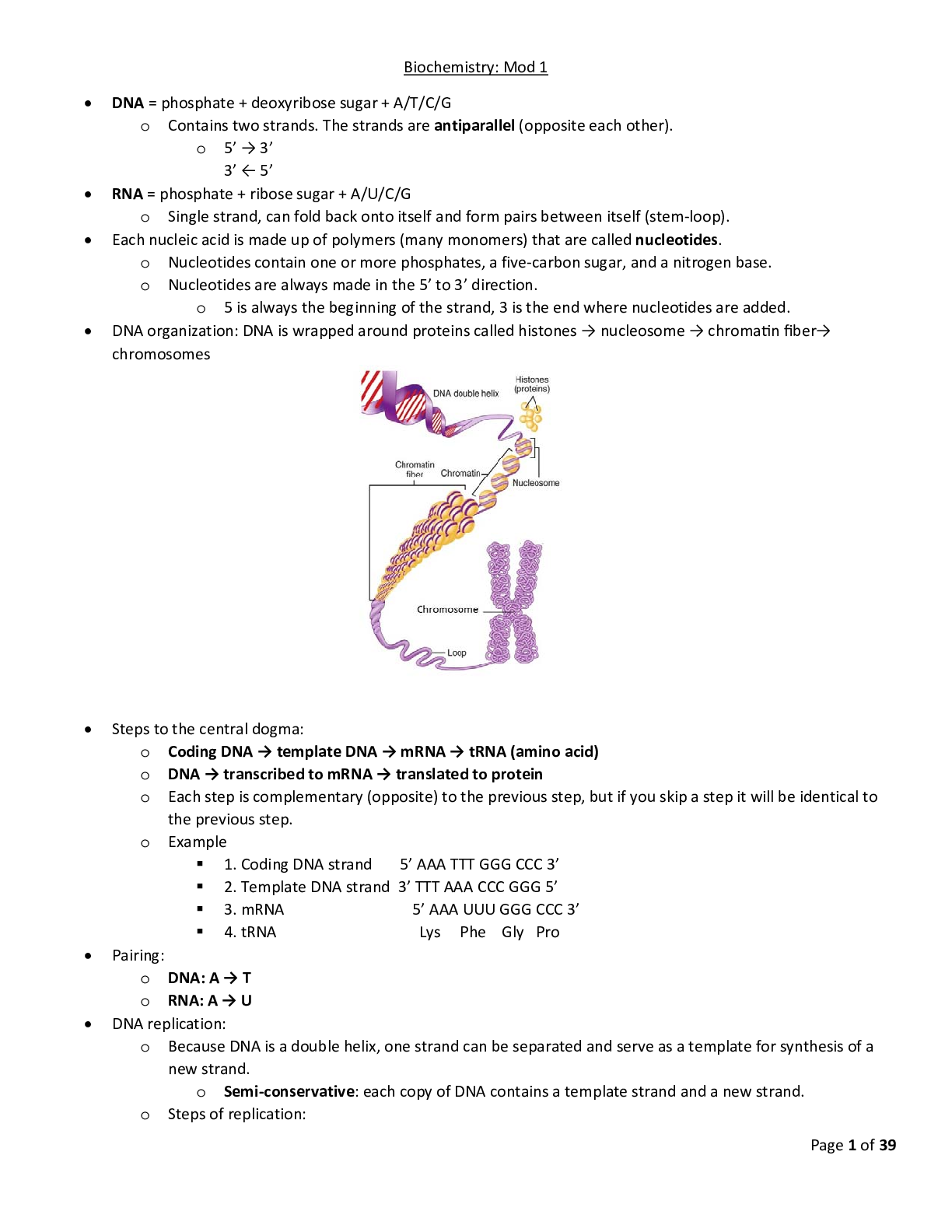
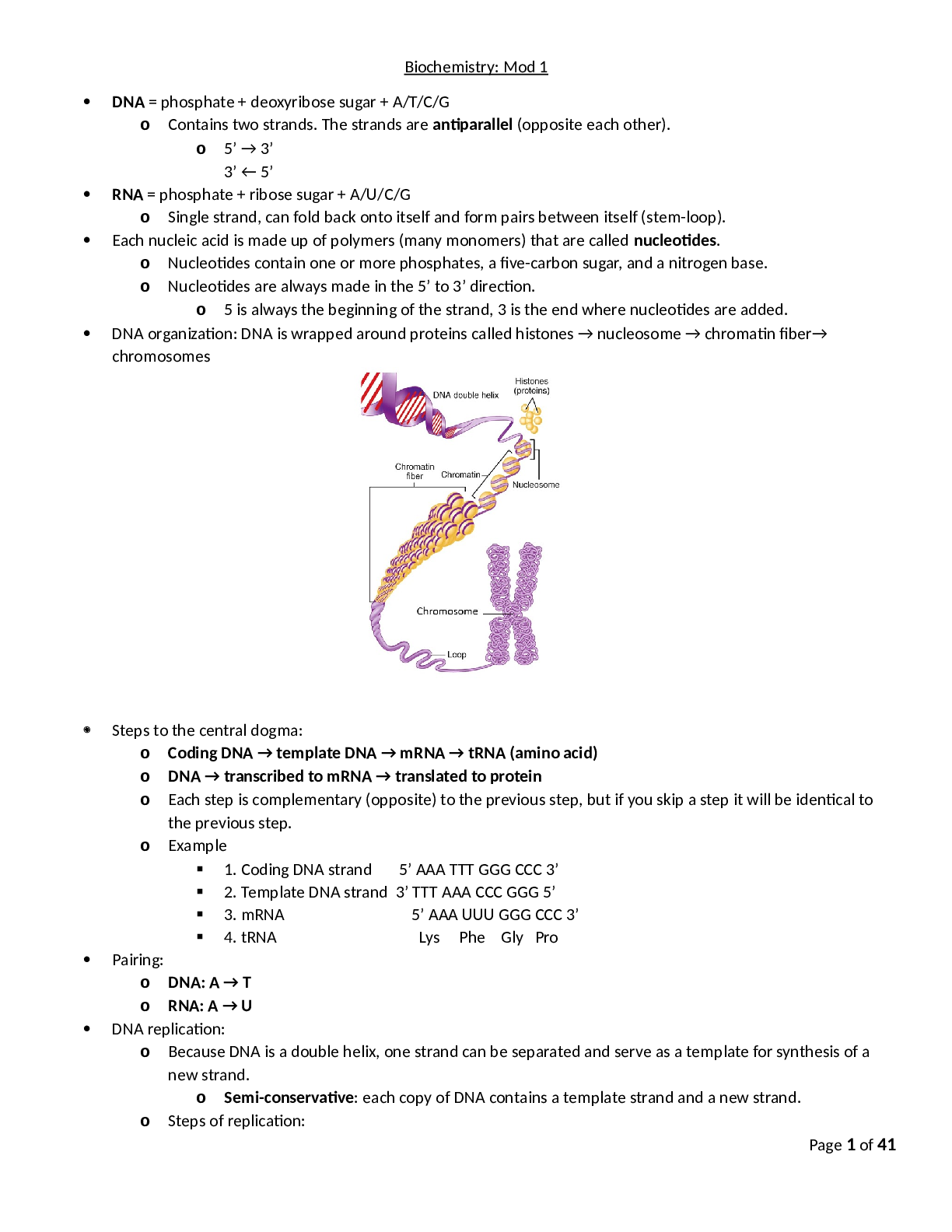
Biochem C785 Kaleys_Comprehensive_Study_Guide_final 2020 Page 1 of 39 Biochemistry: Mod 1 DNA = phosphate + deoxyribose sugar + A/T/C/G o Contains two strands. The strands are antiparallel (oppos... ite each other). o 5’ → 3’ 3’ ← 5’ RNA = phosphate + ribose sugar + A/U/C/G o Single strand, can fold back onto itself and form pairs between itself (stem‐ loop). Each nucleic acid is made up of polymers (many monomers) that are called nucleotides. o Nucleotides contain one or more phosphates, a five‐carbon sugar, and a nitrogen base. o Nucleotides are always made in the 5’ to 3’ direction. o 5 is always the beginning of the strand, 3 is the end where nucleotides are added. - - - - - - - - - - - - - - - - - - - - - - o Exonuclease removes all of the RNA primers, and DNA polymerase fills in those gaps. o DNA ligase seals the two strands forming a double helix. DNA → transcribed → mRNA → translated → protein Transcription occurs in the nucleus: o Initiation: RNA polymerase binds to a sequence of DNA called the promoter, found near the beginning of a gene. Each gene has its own promoter. Once bound, RNA polymerase separates the DNA strands, providing the single‐stranded template needed for transcription.Page 3 of 39 o Elongation: One strand of DNA, the template strand, acts as a template for RNA polymerase. As it "reads" this template one base at a time, the polymerase builds an RNA molecule out of complementary nucleotides, making a chain that grows from 5' to 3'. The RNA transcript carries the same information as the non‐template (coding) strand of DNA, but it contains the base uracil (U) instead of thymine (T). o Termination. Sequences called terminators signal that the RNA transcript is complete. Once they are transcribed, they cause the transcript to be released from the RNA polymerase. o Pre‐mRNA must go through extra processing before it can direct translation. They must have their ends modified, by addition of a 5' cap (at the beginning) and 3' poly‐A tail (at the end). Pre‐mRNAs must also undergo splicing. In this process, parts of the pre‐mRNA (called introns) are chopped out, and the remaining pieces (called exons) are stuck back together.Page 4 of 39 Translation occurs in the cytoplasm: o Initiation: The ribosome assembles around the mRNA to be read and tRNA brings in its perspective protein, decoding 3 bases at a time, beginning with the start codon, AUG. o These 3 base pairs of mRNA are called codons. The mRNA base pairs are complementary to the base pairs of the tRNA, called anticodons. o Elongation: The amino acid chain gets longer. The mRNA is read one codon at a time, and the amino acid matching each codon is added to a growing protein chain. When the complementary pairs are formed, they are added to the protein chain by peptide bonds, the result is polypeptides. o Termination: The finished polypeptide chain is released when a stop codon (UAG, UAA, or UGA) enters the ribosome. Gene regulation o Promotor sites: can be turned off or on, enabling or disabling a gene from being replicated. o Alternative splicing: Exons are used to code for protein, introns are clipped out. The order of exons can determine different mature mRNA strands which result in different proteins. o Epigenetics: involves packaging of DNA. DNA is round around histones. These packages are called nucleosomes. How tightly packed they are determines whether or not the gene is on or off.Page 5 of 39 o Loosely packed = transcription possible. o Tightly packed = transcription impeded. o Modifications determine how tightly/loosely packed they are. Many of these modifications are determined by environment/diet. - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - -- Inheritance o 1‐22 are autosomal chromosomes o If a mutation is on an autosomal chromosome (1‐22) then there is no bias towards males or females. o Sex chromosomes: o Females Xx o Males Xy o X linked mutation is bias because females have Xx and males are Xy. o Allele: a copy of a gene o Genotype: complete set of genes, the genetic makeup of an individual. o Phenotype: all the observable characteristics or traits of an individual, including ones that are not easily seen, such as blood type or color blindness o The genotype (pair of genes) decide the phenotype (observable characteristics) of an individual. o Heterozygous: one allele is dominate while the other is recessive. The dominant allele is observable in the phenotype while the recessive allele is not. (Aa) o Homozygous: two identical alleles (AA or aa) o Dominant: an allele that always expresses its phenotype, even in the presence of a recessive allele, represented by a capital letter. o Recessive: an allele that is only expressed in the phenotype when both alleles of a gene are recessive. Represented by a lower‐case letter. Determining pedigrees o Females are indicated by circles; males are indicated by squares. o Unaffected individuals are indicated by open shapes; affected individuals are indicated by filled shapes. o Recessive vs Dominant o If two unaffected parents have an affected child, they are carrier parents. o Carrier parents = recessive trait o No carrier parents = dominant trait o Autosomal vs Sex linked o Males and females affected equally = autosomal o Only males = sex linked o Autosomal dominant vs sex linked dominantPage 8 of 39 o Affected males with a sex linked dominant trait will pass it on to all of their daughters (females do not inherit father’s Y chromosome, therefore only getting affected X chromosome)Page 9 of 39 Co‐dominance‐ both equally share dominance (red and white) Incomplete dominance‐ neither really stand out (pink) Complete dominance‐ either one or the other is dominant (red OR white)Page 10 of 39 Visual representation with chromosomes Remember that chromosomes come in pairs of two, eliminate the answers that don’t include pairs. Carriers have one of each allele (Rr). If the person actually has the disease, they will have both alleles (rr) or (RR). Autosomal is chromosomes 1‐22, sex linked is chromsomes X and Y.Page 11 of 39 PCR and genetic testing‐ DNA replication in a test tube o What is needed for PCR 1. Template DNA 2. Nucleotides (dNTP’s) 3. DNA polymerase 4. DNA primers o Stages of PCR: 1. Denaturation 95 degrees C Separates the template DNA strands to be able to copy each strand. 2. Annealing 50 degrees C DNA Primers that match the gene were looking for attach to the ends of the piece we want to copy. 3. Elongation 70 degrees C DNA polymerase adds on to the primers, building a copy strand. o Using PCR to detect mutation‐ must copy DNA multiple times (2^n, where n= # of cycles) Make primers that flank the mutation and sequence the product Use primers that stick to the mutation. If the mutation is present, it will stick, if it isn’t present, it won’t stick.Page 12 of 39 Epigenetics is the result of making different proteins and gene expression. It determines what genes function and which do not. o Increased expression means you make more proteins. o Decreased expression means you stop making proteins. o 5 required parts, the first three parts are required to turn a gene on. Promoter‐ Start line for making the protein. Transcription factors‐ foot blocks for the runner (RNA polymerase) to start with. RNA polymerase‐ the runner, makes the mRNA Need all three of the above to turn gene on (increase expression) Nucleosomes‐ the packaging of DNA. Spacing determines whether or not the promoter is visible for a gene to be turned on or off. If it is loosely packed, the promoter is visible and can be accessed by RNA polymerase =Increased expression. If it is tightly packed, the promoter cannot be accessed and therefore the gene will remain turned off= Decreased expression. Methylation‐ CH3 (Methyl) is added to DNA or nucleosomes. Turns gene off by causing nucleosome to become tightly packed. Without it, gene remains on. Acetylation‐ Gene expression is turned on because nucleosomes are widely spaced apart and transcription factors can get in to start transcription.Page 13 of 39Page 14 of 39 Biochem Mod 2 Amino acids o Amino acid back bone‐ same in all amino acids Central carbon (alpha carbon)‐ carbon in the center that holds the amino acid together as other groups bind to it. C‐H, CH Amino group‐ contains nitrogen and hydrogen. NH2, NH3+ Carboxyl group‐ has two oxygens and one carbon, gives the amino acid its acid properties. COO‐, COOH o Side groups (R group) are different which effect how the amino acid acts, will classify them as charged, polar, or hydrophobic. To determine what their classification is, first look for: Charged amino acids‐ R group will have ‐/+ which means negatively or positively charged. If charged, will take precedence. If not charged, look to see if its polar. Polar amino acids‐ will have SH, OH, NH at the end of the R group. If not polar, then the amino acid is non‐polar. Non‐polar amino acids‐ will have CH at the end of the R group. Hydrophobic amino acids‐ will have several hydrogen molecules in the R group, each will end with an H atom. o Bonds Amino acids will have three types of bonds Charged amino acids make ionic bonds (only + with ‐) Polar amino acids will make hydrogen or disulfide bonds o OH and NH make hydrogen bonds o SH makes disulfide bond (the strongest type of bond, can only bond with itself so very few of them) o “OH look it’s a Northern Hemisphere/Southern Hemisphere Polar Bear!” Non polar amino acids will make hydrophobic interactions o CH “Can’t have water” (weakest bond, but many of them) Strongest to weakest: disulfide, ionic, hydrogen, hydrophobic What breaks the bonds (denaturing) Charged: pH and salt changes Polar: pH and salt changes, disulfide bonds have to be broken by reducing agents Non polar: broken by heatPage 15 of 39 Alanine: be able to recognize structure. Is hydrophobic and ionized (will have + or ‐)Page 16 of 39 Protein structure‐ chains of amino acids o Linking amino acids together‐ Forming peptide bonds (the backbone of an amino acid) Primary structure: chain of amino acids by peptide bonds, does not denature The carboxyl group and amino group of two amino acids bond together by using 2 hydrogens and one H2O. A water molecule is lost in this process, known as dehydration. o Amino group + carboxyl group Secondary structure: shaped that is formed when hydrogen bonds are added between carboxyl and amino groups. Forming of alpha helix and beta sheets within the backbone, held together by hydrogen bonds. Tertiary structure: three‐dimensional folding, the result of different secondary structures interacting with one another via their R groups/side chains. These interactions include hydrophobic interactions, hydrogen bonds, ionic bonds, and disulfide bonds. Proteins can now function at this stage. Disruption of its hydrophobic state is the simplest way to denature. Quaternary structure: more than one amino acid/polypeptide/protein, held together by R groups/side chains. Not all proteins need this structure.Page 17 of 39 Protein folding o Chaperones help fold proteins o Can misfold, or take another shape (conformation), results in it being non‐functional o Denature: environmental change that causes the protein to misfold or unfold by breaking side chain bonds/secondary structure, but does NOT break the primary structure o Degradation: breaking apart of the primary structure/peptide bonds by hydrolysis o Aggregation: proteins clump together abnormally due to hydrophobic interactions either by unfolding or mutation. o Hydrophobic interactions: when a protein is exposed by unfolding, causing its hydrophobic parts to be exposed to water o Misfolding of proteins leads to Alzheimer’s: Intracellular tangles and extracellular plaques (senile plaques) are caused by aggregated amyloid‐beta fibers which accumulate in the brain. Connections between tau is lost leading to progressive neurodegeneration.Page 18 of 39 Enzymes o One of the most important types of proteins in our cells. o Known as catalysts, they speed up reactions and use less energy ↑rate of reacƟon, ↓acƟvaƟon energy Can do the same reaction over and over They only act on specific substrates‐ a molecule that is specific for that enzyme Binds via its active site, a binding platform for its specific substrate Have a high degree of specificity‐ they catalyze only one type of reaction, and most act on only one substrate Molecules that are different in shape or function bind to the enzyme. Therefore, the enzyme and its substrate are complementary, like a “lock and key”. While an enzyme and its substrate are complementary, many enzymes will adjust their active site slightly to improve the fit of the substrate. Known as induced fit, “hug”. o Reusable‐ Enzyme cycle Enzyme + specific molecule/induced fit = reaction/product, then release of product Enzyme pathways o Enzyme reaction: substrate + enzyme = product o Enzyme pathway: Substrate + enzyme 1 = product → Enzyme 1 product (now substrate) + enzyme 2 = enzyme 3 The product of one enzyme can be the substrate of another. o Control of enzyme activity An organism must be able to control its enzyme availability and activity. 1. Control of enzyme availability‐ depends on its rate of synthesis and degeneration. Changes over time. 2. Control of enzyme activity‐ can be inhibited due to too much product or presence of inhibitors, and can be modulated (modified) through structural alterations. o Control by modification: phosphorylation and dephosphorylation (attachment/removal of a phosphate group)Page 19 of 39 o Control by enzyme inhibition: Feedback inhibition – similar to the drug induced form of noncompetitive inhibition, when excess product is detected, the pathway is stopped by inhibition. The product at the end of the pathway binds to allosteric site on the first enzyme at the beginning of the pathway to stop the process. o Medical inhibition with drugs Competitive inhibition‐ appears similar to the substrate, binds to the active site on the enzyme to prevent substrate from binding Can be overcome by adding additional substrate, if there’s too much substrate and not enough inhibition the substrate will win. Noncompetitive inhibition‐ binds to the allosteric site (away from the active site) Changes shape of protein and therefore changes the active site, causing the substrate to not be able to fit into the enzyme.Page 20 of 39 Biochem Mod 3 - - - - - - - - - - --- - - - - - - - - - - - - -he electrons, oxygen combines with protons to create water. The role of oxygen is crucial for the generation of ATP in the cell and is the sole use of the oxygen we breathe. ATP synthase uses the proton gradient created by the electron transport chain to generate ATP from ADP and phosphate in a process known as oxidative phosphorylation.Page 27 of 39 The electrons are passed throughout the chain via each protein complex (as illustrated, left to right) Protein complex 1 (1st complex) takes NADH, passes to protein complex 2, which accepts it as FADH2, etc. The energy passed between complexes moves protons (H+) across the membrane into the intermembrane space (proton gradient). Only complexes 1, 3 and 4 can pump protons. Complex 2 can only pass electrons to the next complex. H+ evens itself out on either side by going through the channel of ATP synthase (as illustrated on the right). 30 ATP are made per 1 glucose. H+ goes through channel, turns into ATP. Oxygen is the final electron acceptor. Without oxygen (anaerobic) the electrons have no where to go and they begin to back up, which in turn causes the citric acid cycle to back up as well. The mitochondria shuts down and changes to anaerobic metabolism mode. Anaerobic Metabolism:Page 28 of 39 o When there is no oxygen available, cells can convert pyruvate to lactate under anaerobic conditions to allow glycolysis to continue producing some ATP. This is done via fermentation. Two hydrogen atoms are added to pyruvate to form lactic acid (lactate). Formation of lactate regenerates the NAD+ that was used during glycolysis, thus allowing glycolysis to continue making small amounts of ATP for the cell. Lactate is eventually converted back to glucose via gluconeogenesis when the cell is no longer without oxygen. This whole process is known as the Cori Cycle. o The Cori Cycle Glycolysis → pyruvate → fermentaƟon → lactate → gluconeogenesis in liver (uses 6 ATP) → glucose → glycolysis Inefficient because uses 6 ATP to produce 2 ATP Takes place in muscles when over worked, and in the red blood cell because they have no mitochondria. Gluconeogenesis—when the cell has ample ATP it shifts from making ATP to storing it as glucose or glycogen via glucogenesis‐ essentially the opposite of glycolysis. Gluconeogenesis takes excess pyruvate and converts it back into glucose for storage.Page 29 of 39 IN OUT +Net gain Glycolysis Glucose 2 ATP 2 NAD+ 4 ADP 2 pyruvate 4 ATP 2 NADH 2 ADP 2 pyruvate 2 ATP Citric Acid Cycle Acetyl CoA NAD+ FAD H+ CO2 NADH FADH2 ATP The Cori Cycle Pyruvate NADH H+ 6 ATP Lactate NAD+ 2 ATP Loss of 4 ATP Electron transport chain NADH FADH2 NAD FAD ATP + 30 ATPPage 30 of 39 G word break down: o Glyco/gluco = glucose o Glycogen = stored glucose o Genesis= make o Lysis= break o Neo= new G words o Glucose= sugar o Glycogen‐ stored glucose o Glycolysis‐ break down glucose to make ATP o Gluconeogenesis‐ make new glucose o Glycogenesis= make stored glucose in the liver for short term use o Glycogenolysis‐ break down (stored) glycogen into new glucose o Glut4= door that allows insulin into cells G words and their processes: o Insulin is released when there’s high glucose in the blood. Lets glucose into cells, signals glut4 to open its door to allow glucose into cell. 1. Once in the cell‐ glycolysis= glucose → pyruvate → ATP (energy) 2. If too much glucose, short term storage in liver 3. Fatty acid synthesis‐ make fat from acetyl CoA, stored in triglycerides within adipose tissue o Glucagon= released when there’s low glucose, (glucose is gone) Glycogenolysis‐ break glucose out of glycogen Gluconeogenesis‐ make new glucose from Lactate Acetyl CoA → pyruvate →glucose Glycerol (breakdown fats) Amino acids (proteins, as last resort) Beta Oxidation‐ break fats into acetyl CoAPage 31 of 39 Biochem Mod 5 Fatty acids‐ the simplest of lipids. All lipids are nonpolar and hydrophobic. o Alpha, beta, omega bonds Alpha is attached to carboxyl Beta is attached to alpha‐ breaks when we break down fatty acid for energy (beta oxidation) Omega is complete opposite (end) of carboxyl, count left to right from omega carbon to determine which # carbon is the double bond o Fluidity More double bonds always mean more fluid (except trans) If they have the same number of double bonds, shortest chain has the most fluid Saturated fatty acid‐ solid at room temp, from animal sources (ie. butter) No double bonds between carbons Straight or linear, long chain, stays compacted together, can stack well Contain maximum number of hydrogens Unsaturated fatty acid‐ oils that are liquid at room temp, plant based At least one carbon that is not paired with hydrogen Has at least 1 double bond between carbons Short chain, kinked, can move around easily, doesn’t stack well (d/t double bonds) Lose 2 hydrogens per double bond Unsaturated fatty acids melt at cooler temperatures than saturated fatty acids of the same chain length Monounsaturated fatty acid‐ A fatty acid containing one double bond Polyunsaturated fatty acid‐ A fatty acid with more than one double bond in its carbon chainPage 32 of 39Page 33 of 39 o Essential fatty acids‐ Must be eaten in diet. There are different categories of unsaturated fatty acids depending on the location of the first double bond in the carbon chain. If the first double bond occurs between the third and fourth carbons, counting from the omega end (CH3) of the chain, the fat is said to be an omega‐3 fatty acid (EPA/DHA, fish oil) If the first double bond occurs between the sixth and seventh carbons (from the omega end), the fatty acid is called an omega‐6 fatty acid; this type of fatty acid is obtained from vegetable oils such as corn and safflower oil. Omega 3, Omega 6 have to be eaten in our diet‐ essential Omega 9 is not essential, body can make it o Rules for fatty acids All fatty acids only have 2 oxygens If it is saturated, there will be twice as many hydrogens as carbons. If unsaturated, every double bond loses 2 hydrogens. More double bonds = more fluid, if they have the same # of double bonds then the shorter chain has more fluid. o Cis vs trans Cis fats: have both hydrogen atoms on the same side of the double bond Have a kink or bend in the carbon chain, making it difficult for the fatty acids to pack together Unsaturated natural oils Trans fats: when the hydrogen atoms are on opposite sides of the double bond Trans fatty acids can pack tightly like saturated fatty acids so they have a higher melting point Has double bonds but acts saturated Unsaturated fats treated with chemicals (margarine)‐ body cannot process When consumed, trans fats raise blood cholesterol levels and increase risk of heart disease.Page 34 of 39Page 35 of 39 Lipid structure and function o Fatty acid structures Four types: 1. Full structure 2. Structural formula 3. Zig‐ zag structures‐ every peak represents a carbon 4. Chemical formula Counting carbons: Shaded yellow is omega carbon group. Count from it to the right until you reach the double bond to determine its omega number. This example is an omega 6 fatty acid. Structural formula: Parentheses mean that that group is repeated, the number to its immediate right is how many times. In this example, CH2 is repeated 4 times. Zig zag structure: Each point on the zig zag structure represents a carbon. If any of the carbons are missing their bonds, you can assume that the remaining bonds not shown are to hydrogen.Page 36 of 39 Sterol family‐ lipids with 4 rings, include cholesterol and testosterone. Be able to recognize them. o Cholesterol is needed to synthesize vitamin D in the skin; cholic acid, a component of bile; and steroid hormones. o The steroid hormones include testosterone and estrogen, which promote growth and the development of sex characteristics, and cortisol, which is released in response to stress and promotes glucose synthesis in the liver Triglycerides‐ fat and oils within the body, consist of a backbone of glycerol with three fatty acids. o Glycerol + 3 fatty acids = triglyceride o Two glycerols can make glucose o If only one fatty acid is attached to the glycerol, the molecule is called a monoglyceride o When two fatty acids are attached, it is a diglyceride. o Triglycerides store energy‐ fatty acids can be oxidized to produce ATP. If the body has no immediate need for energy, they are stored as triglycerides in adipose tissue and in the liver. Consist of 98% of our energy reserves.Page 37 of 39 Vitamins‐ fat soluble, must have some sort of fat in order to be absorbed. Recognize them‐ they do not have 4 rings o Vit A: for the eyes o Vit K: blood clotting o Vit E: antioxidant o Vit D: promotes absorption of calcium Arachidonic acid is the precursor to many eicosanoids. o Eicosanoids are another class of lipids derived from essential fatty acids that also act as local hormones. Generated as needed Are involved in the production/regulation of pain and fever, blood pressure, blood coagulation, and reproduction. Treatment with aspirin inhibits the production of PGH2, which decreases the production of several eicosanoids involved in the development of inflammation, fever, and blood clotting.Page 38 of 39 Phospholipid structure and function o Have a backbone of glycerol with two fatty acids attached, as well as a phosphate group which attaches to a variety of other molecules Glycerol + two fatty acids + phosphate group Fatty acid end is hydrophobic (lipid soluble) The phosphate end is hydrophilic (water soluble) o Considered amphipathic‐ are both lipid and water soluble. o The lipid bilayer‐ phospholipids have polar heads that interact with the watery environment and nonpolar tails that remain inside together to avoid water. Can form tightly packed bilayers, where fatty acids and triglycerides cannot and are leaky. Controls the way things go in and out of cell. Things that control membrane fluidity: number of double bonds (more = fluid, less = solid) cholesterol length of tail (longer = solid, shorter = fluid) Fatty acid synthesis‐ takes place in the cytoplasm o Acetyl‐CoA from digestion is intercepted before it goes through the citric acid cycle. It is taken out of the mitochondria to build fat. o Acetyl‐CoA cannot leave the mitochondria by itself, must pair with oxaloacetate. o Acetyl‐CoA + oxaloacetate = citrate → crosses into cytosol → unjoin, releases Acetyl‐CoA o Acetyl‐CoA is made of two carbons, attached to coenzyme A. o Linking Acetyl‐CoA together, process repeats over and over:Page 39 of 39 Activation by biotin (below picture) then malonyl + Acetyl‐CoA = Butyryl Lipid Metabolism Beta Oxidation‐ the breakdown of fats by breaking the beta bond that occurs in the mitochondria. o Fats are stored as triglycerides‐ three fatty acids attached to triglycerol o Beta oxidation is a major source of cellular energy, especially during a fast when carbohydrates are not available. o Each round of beta oxidation produces one FADH2, one NADH, and one acetyl‐CoA Ins: FAD, NAD+, Coenzyme A, fatty acid Outs: 2 carbon shorter fatty acid chain, acetyl CoA, FADH2, NADH o The citric acid cycle oxidizes the acetyl‐CoA to produce an additional three NADH, one FADH2, and one GTP, further oxidation occurs in the electron transport chain, making the yield of ATP much higher than that of glucose. o To determine the # of acetyl CoA made: # of carbons divided by 2 o To calculate number of rounds “chopping down a tree”: # of acetyl CoA ‐ 1, or # of carbons divided by 2 – 1. o A defect in the enzyme used in beta oxidation is called medium‐chain acyl‐CoA dehydrogenase deficiency (MCADD), which usually presents in infants as vomiting and/or lack of energy due to their inability to utilize medium‐chain fatty acids. The disease is often fatal if not detected early, and newborn infants are routinely screened for this disease. Individuals with MCADD must avoid fasting for prolonged periods and need diets rich in slow‐release carbohydrates to maintain their energy levels.Filename: Kaleys comprehensive study guide final Directory: C:UsersKaley DuniganDocumentsbsn Template: C:UsersKaley DuniganAppDataRoamingMicrosoftTemplatesN Title: Subject: Author: Kaley Dunigan Keywords: Comments: Creation Date: 7/16/2019 7:45:00 PM Change Number: 41 Last Saved On: 8/9/2019 2:20:00 AM Last Saved By: Kaley Dunigan Total Editing Time: 13,206 Minutes Last Printed On: 8/29/2019 8:24:00 AM As of Last Complete Printing Number of Pages: 39 Number of Words: 5,419 (approx.) Number of Characters: 30,891 (approx.) [Show More]
Last updated: 1 year ago
Preview 1 out of 40 pages
Reviews( 0 )
Document information
Connected school, study & course
About the document
Uploaded On
Dec 17, 2020
Number of pages
40
Written in
Additional information
This document has been written for:
Uploaded
Dec 17, 2020
Downloads
0
Views
30

 Rasmussen College.png)





















.png)



