Chemistry > QUESTIONS & ANSWERS > CHEM101 Topic 13: Acids and Bases[ALL ANSWERS 100% CORRECT] (All)
CHEM101 Topic 13: Acids and Bases[ALL ANSWERS 100% CORRECT]
Document Content and Description Below
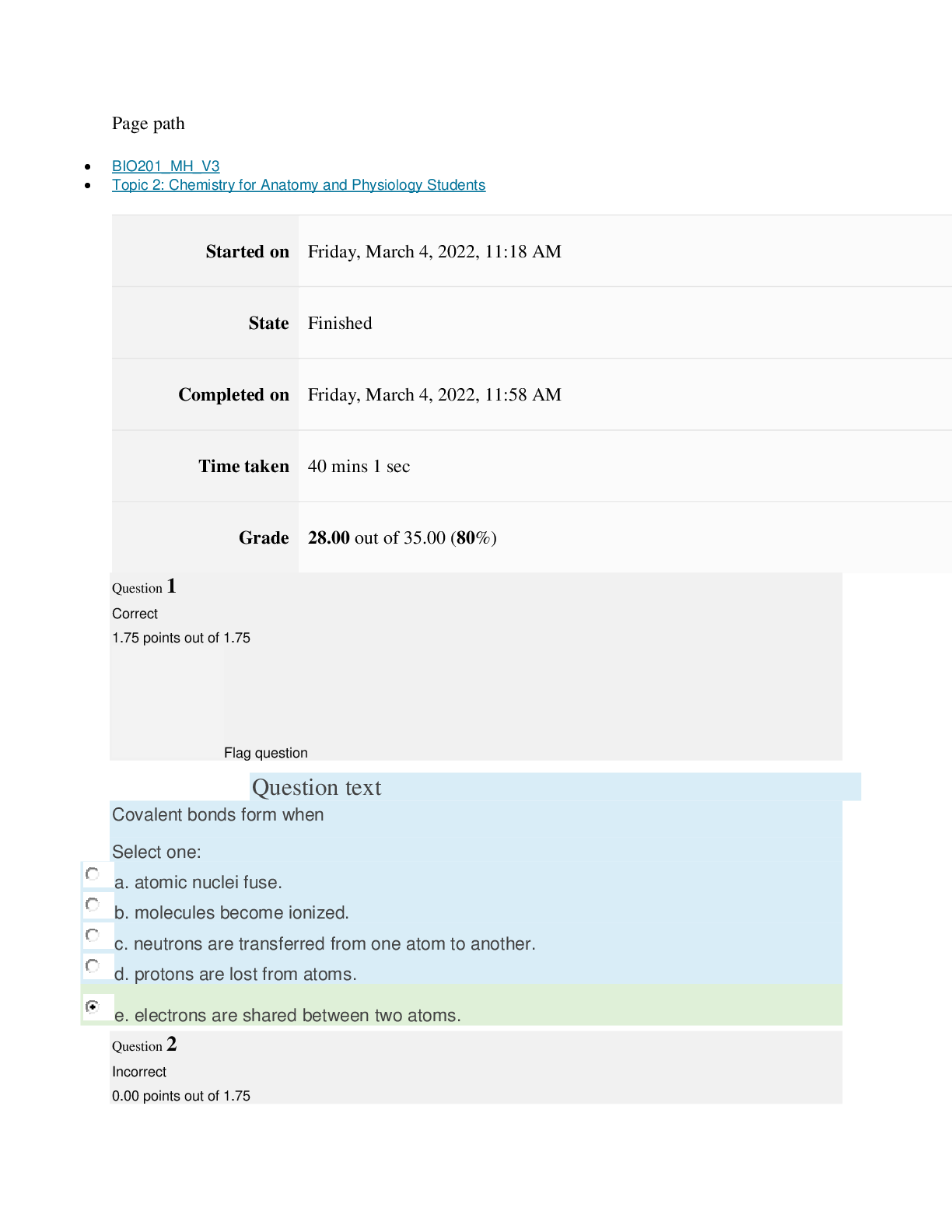
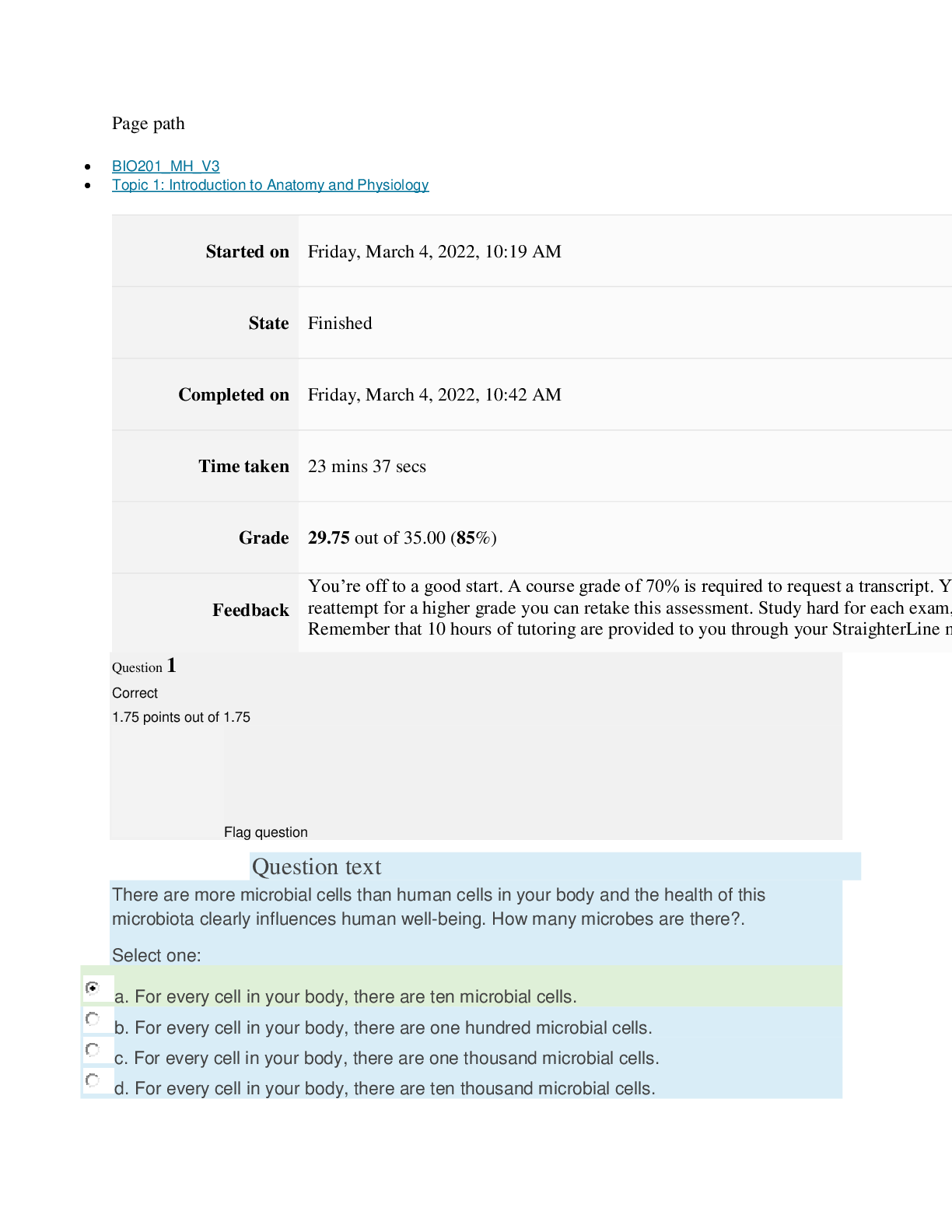
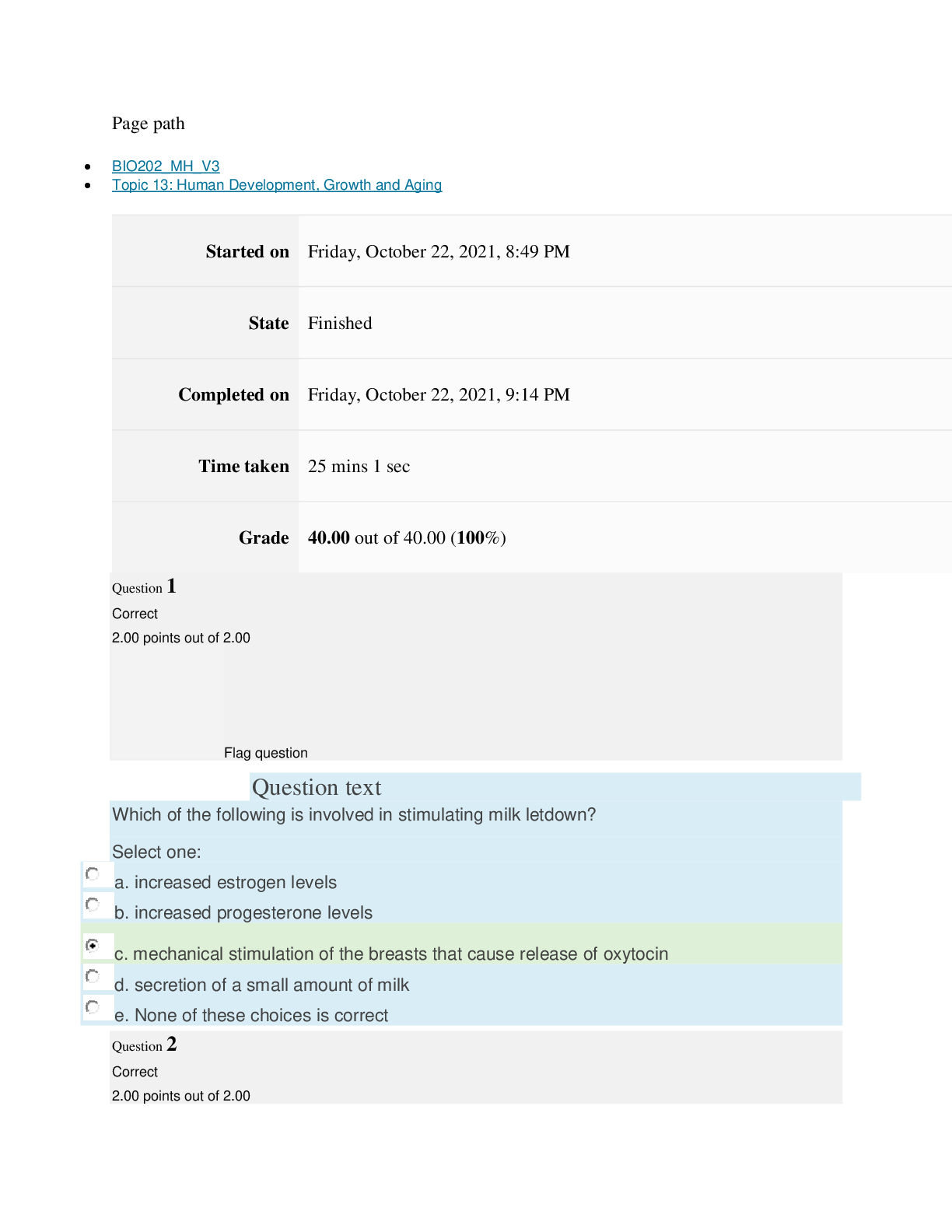
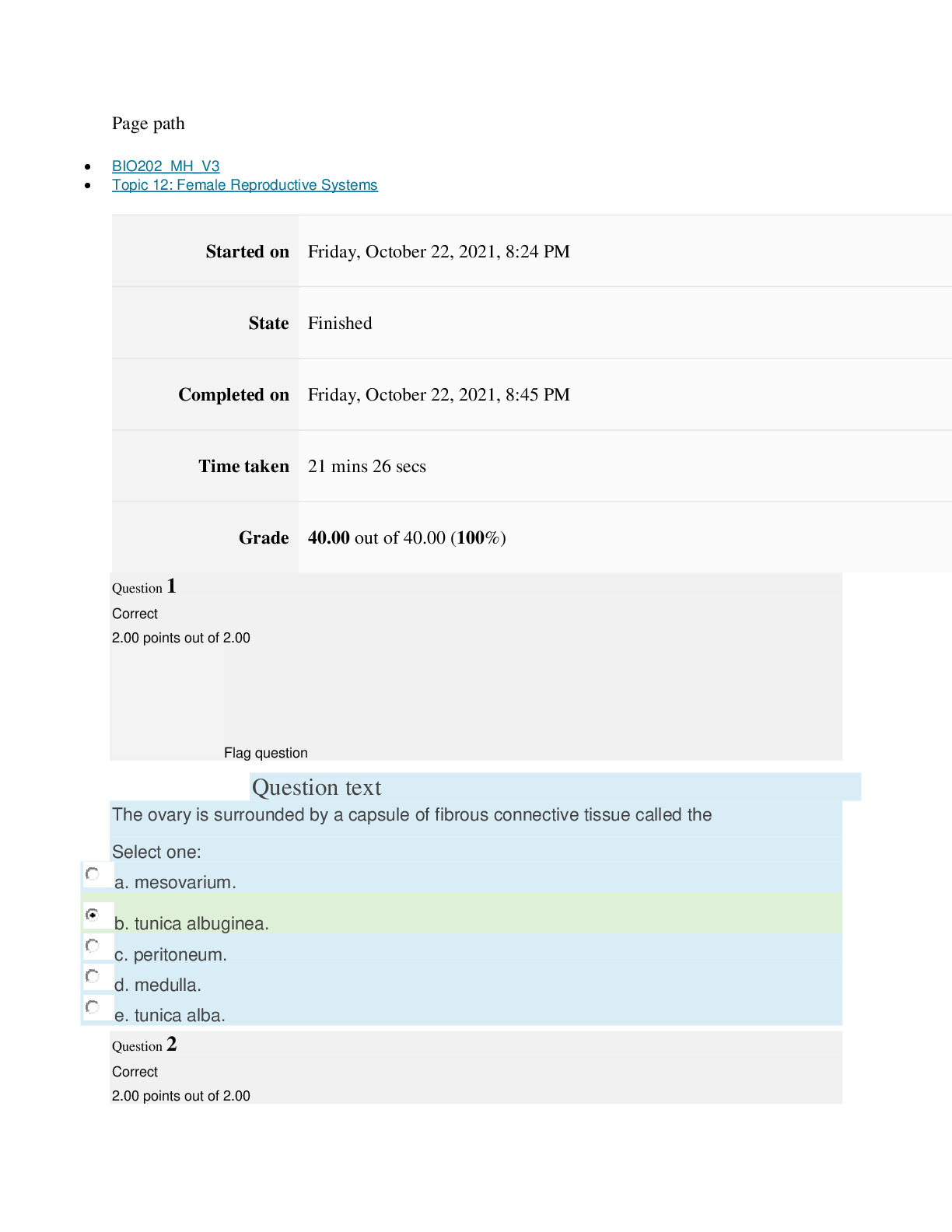
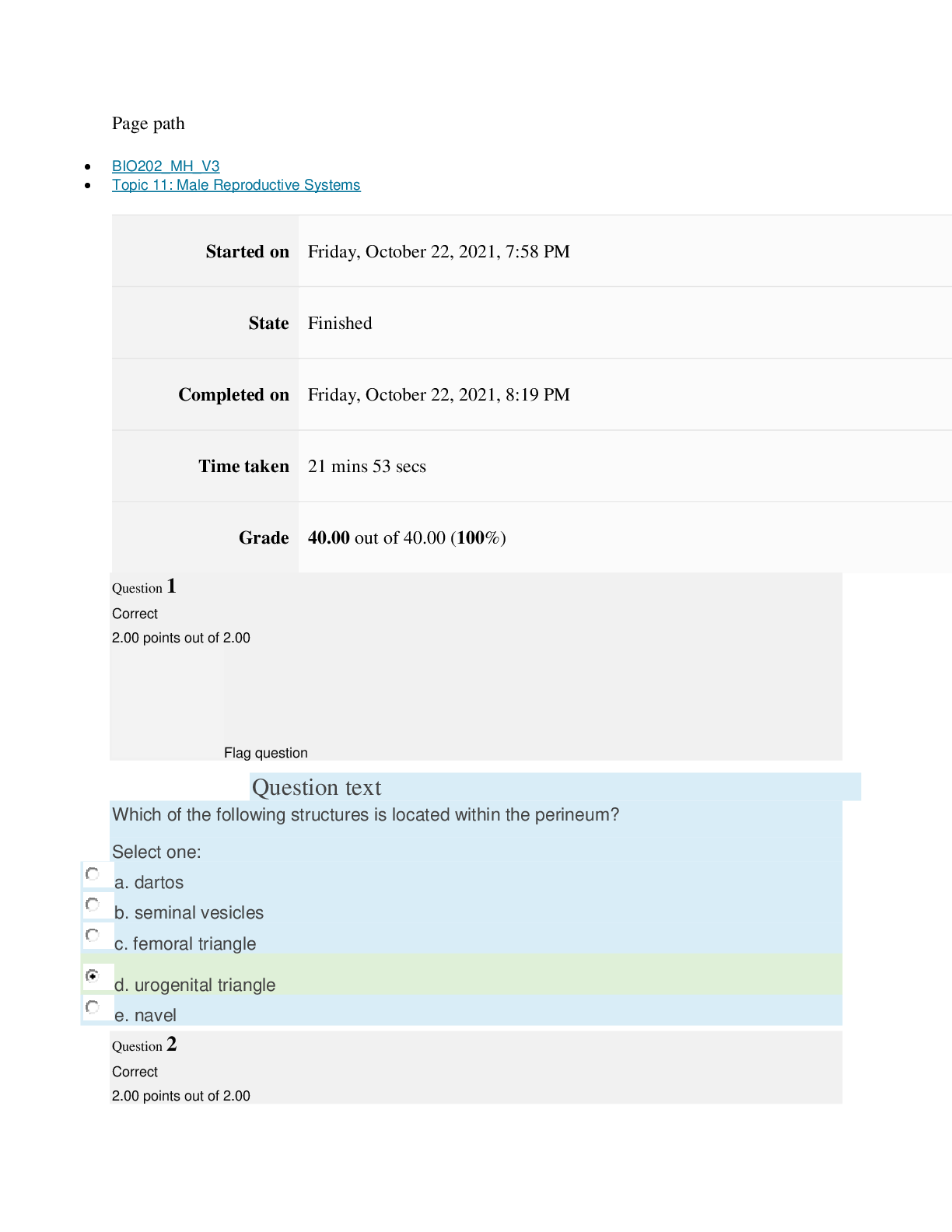
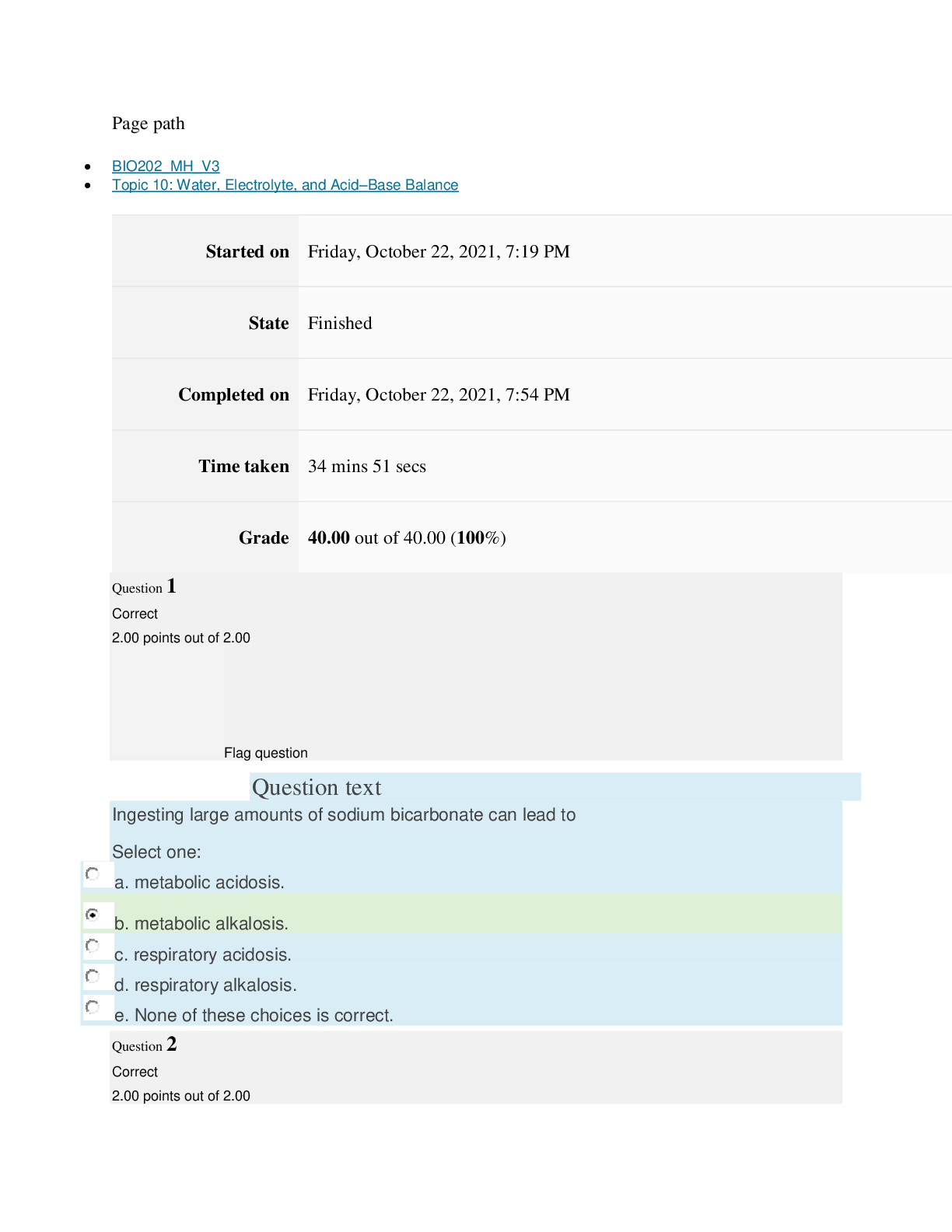
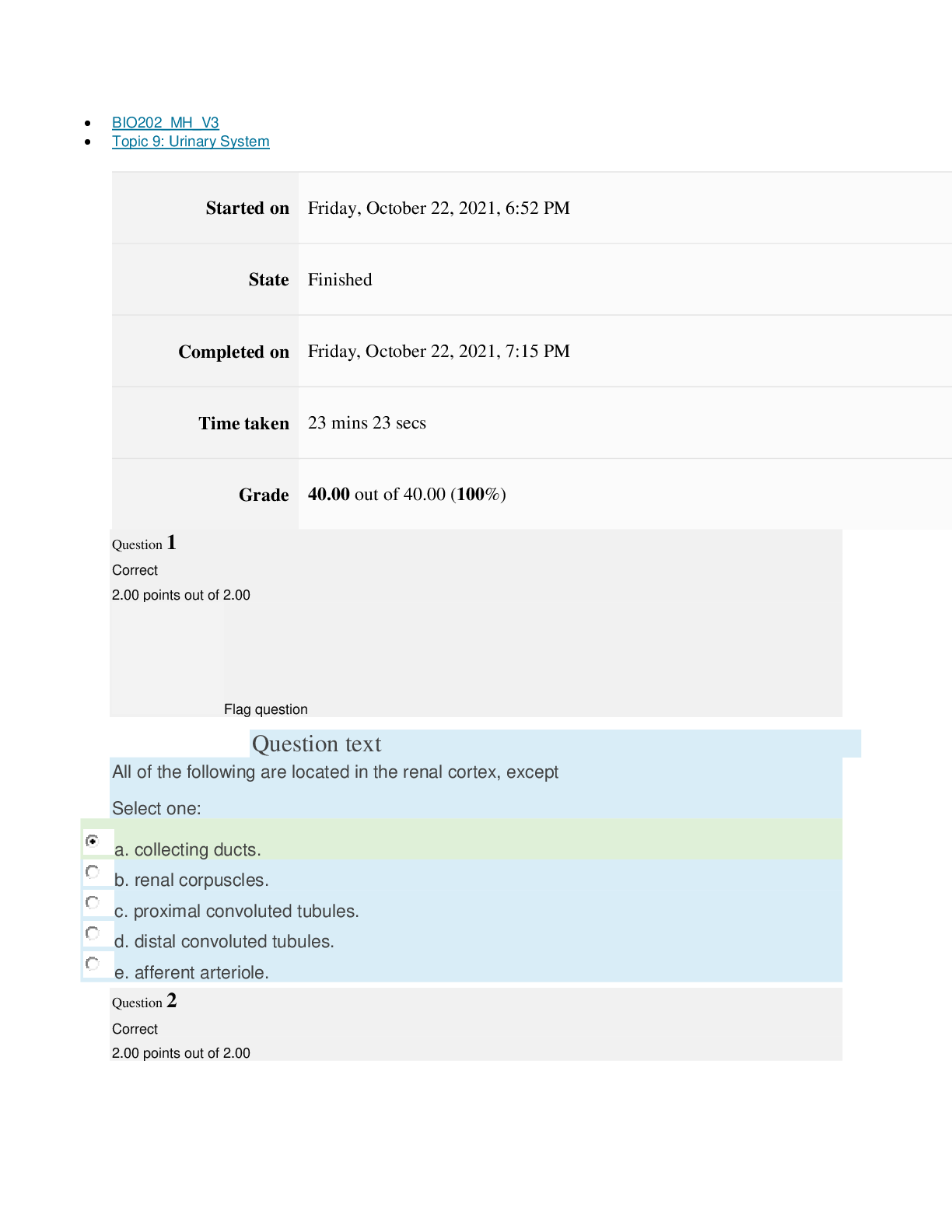
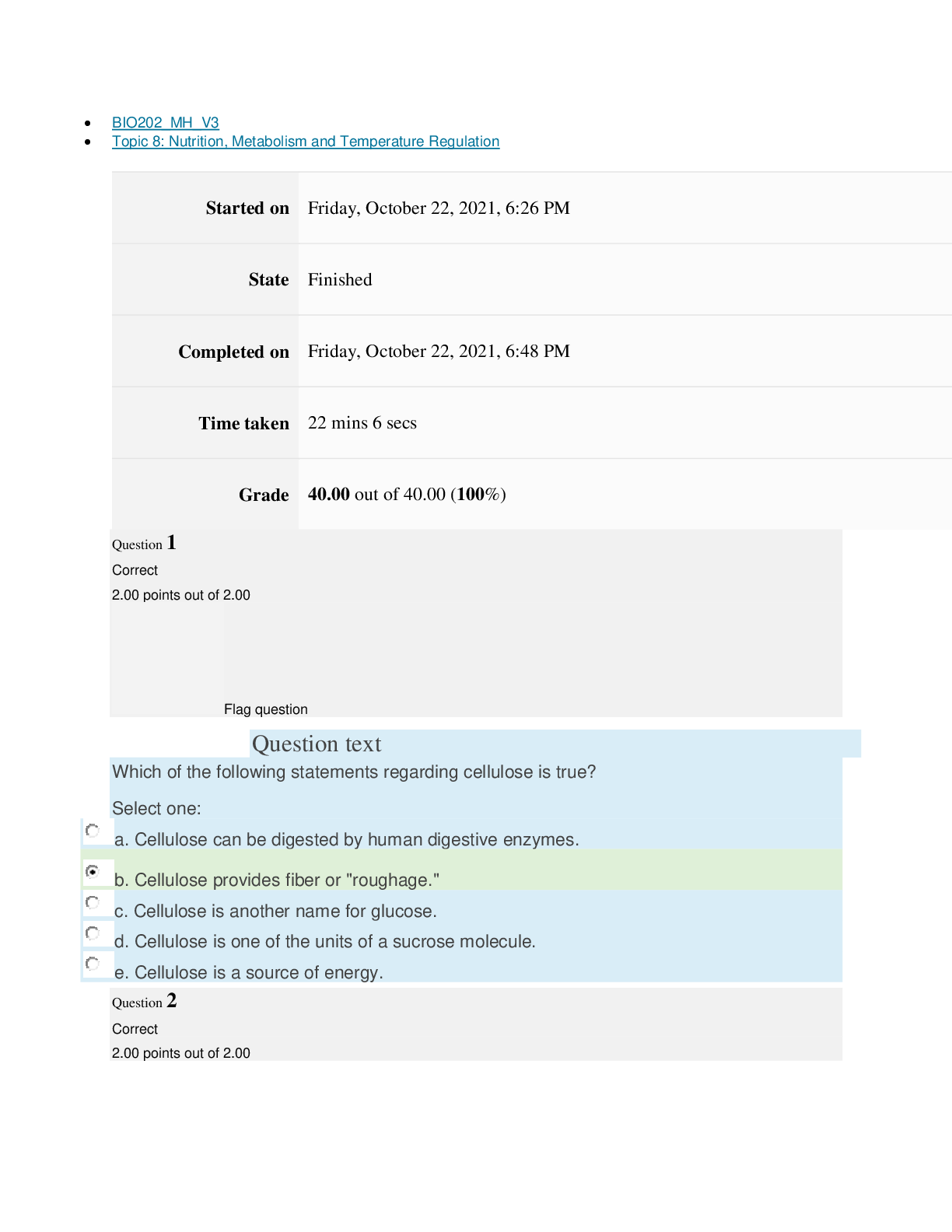
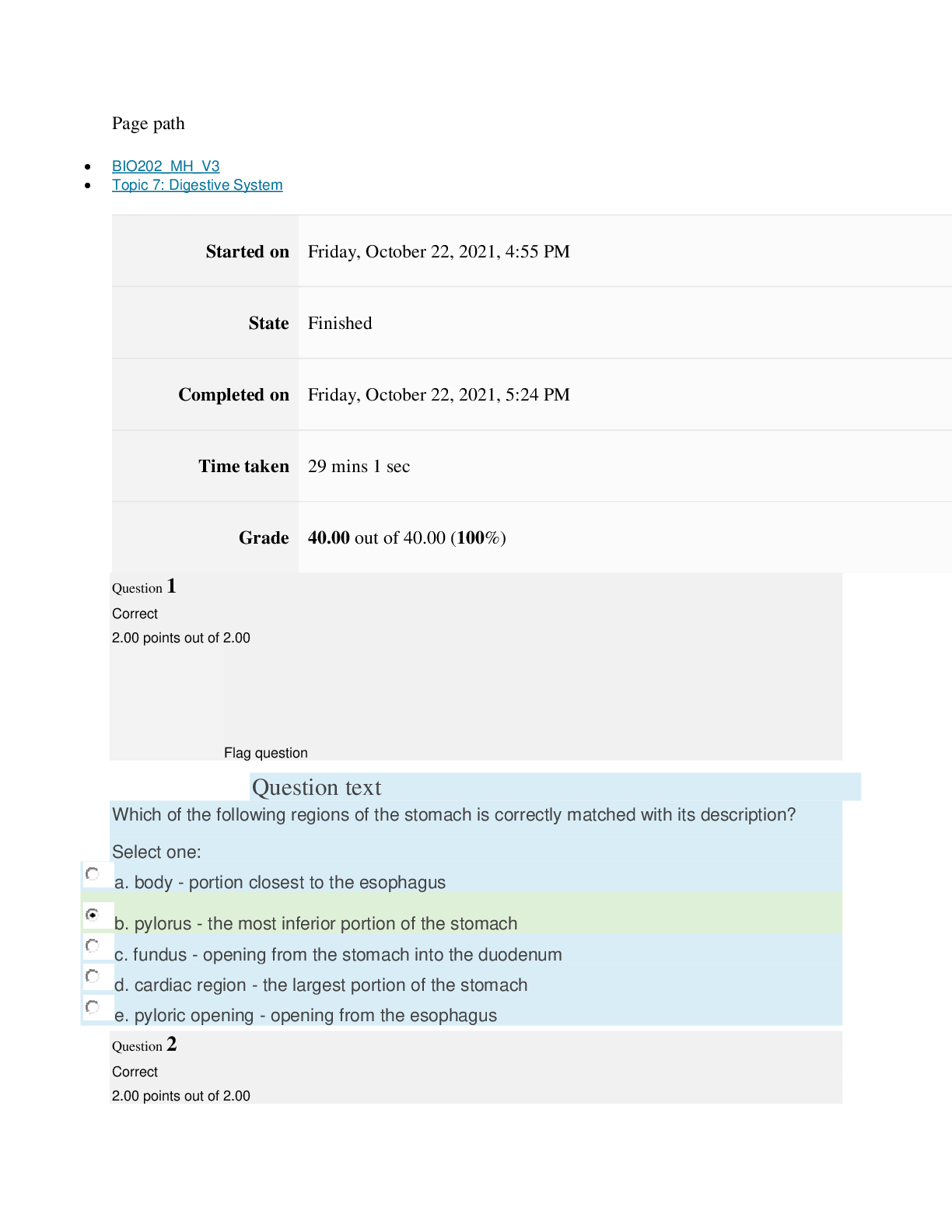
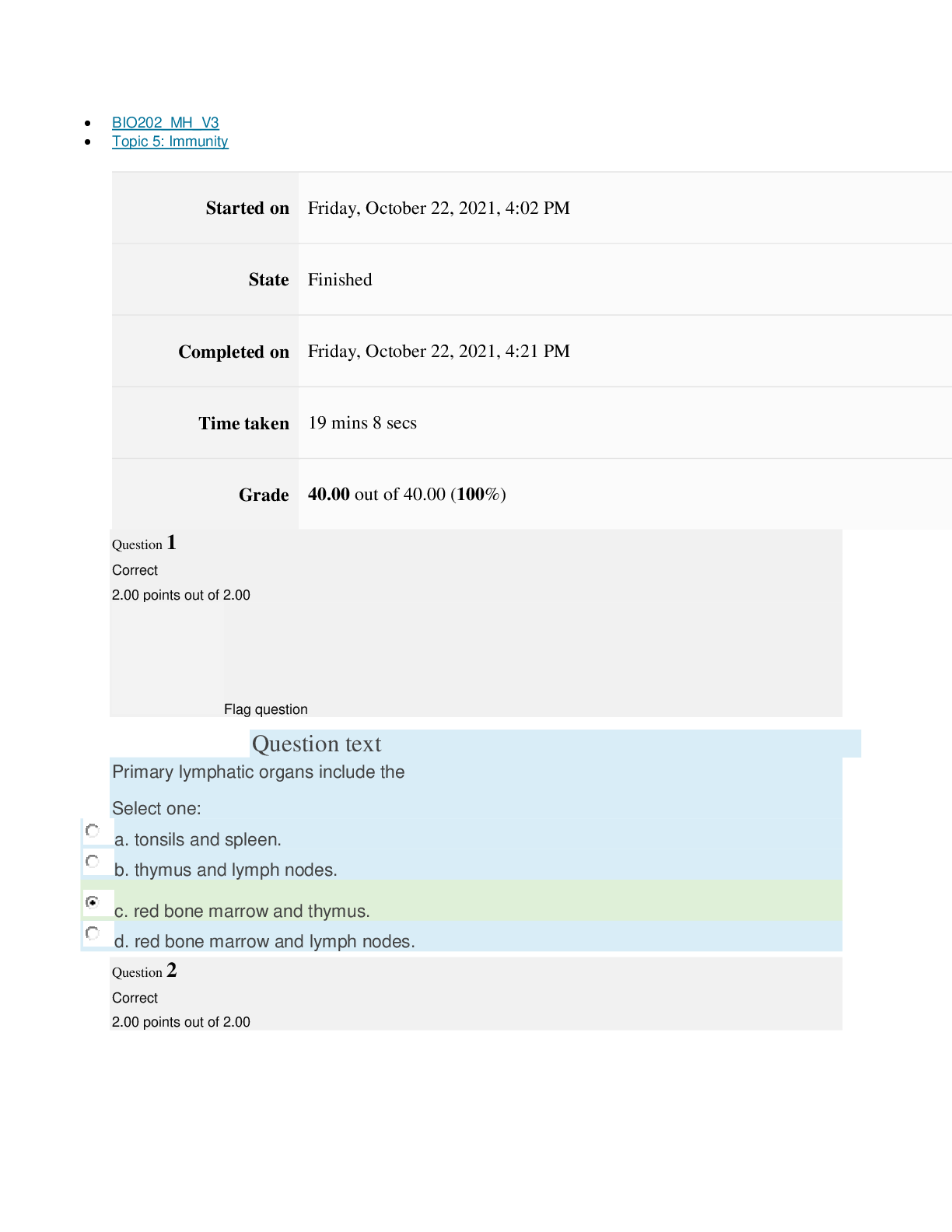
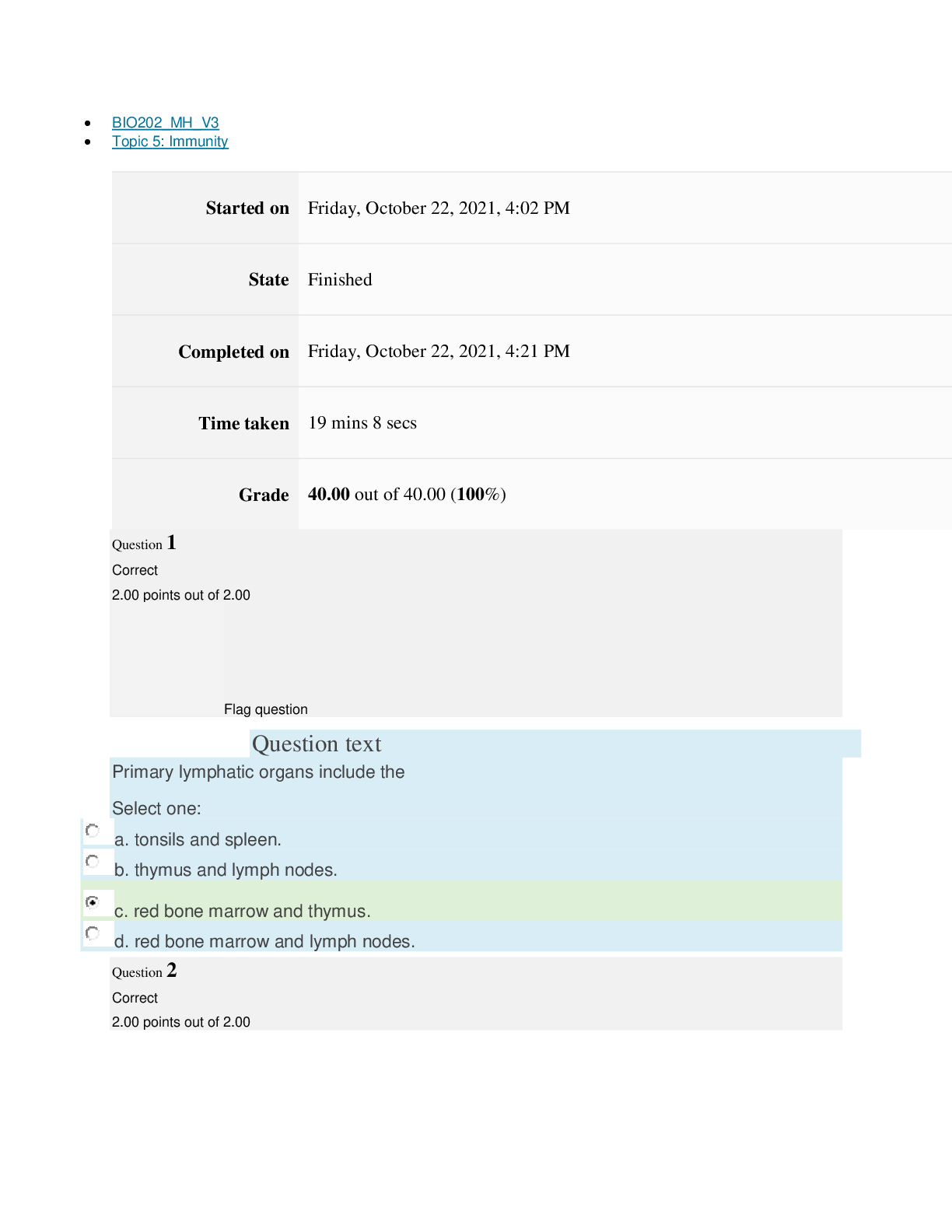
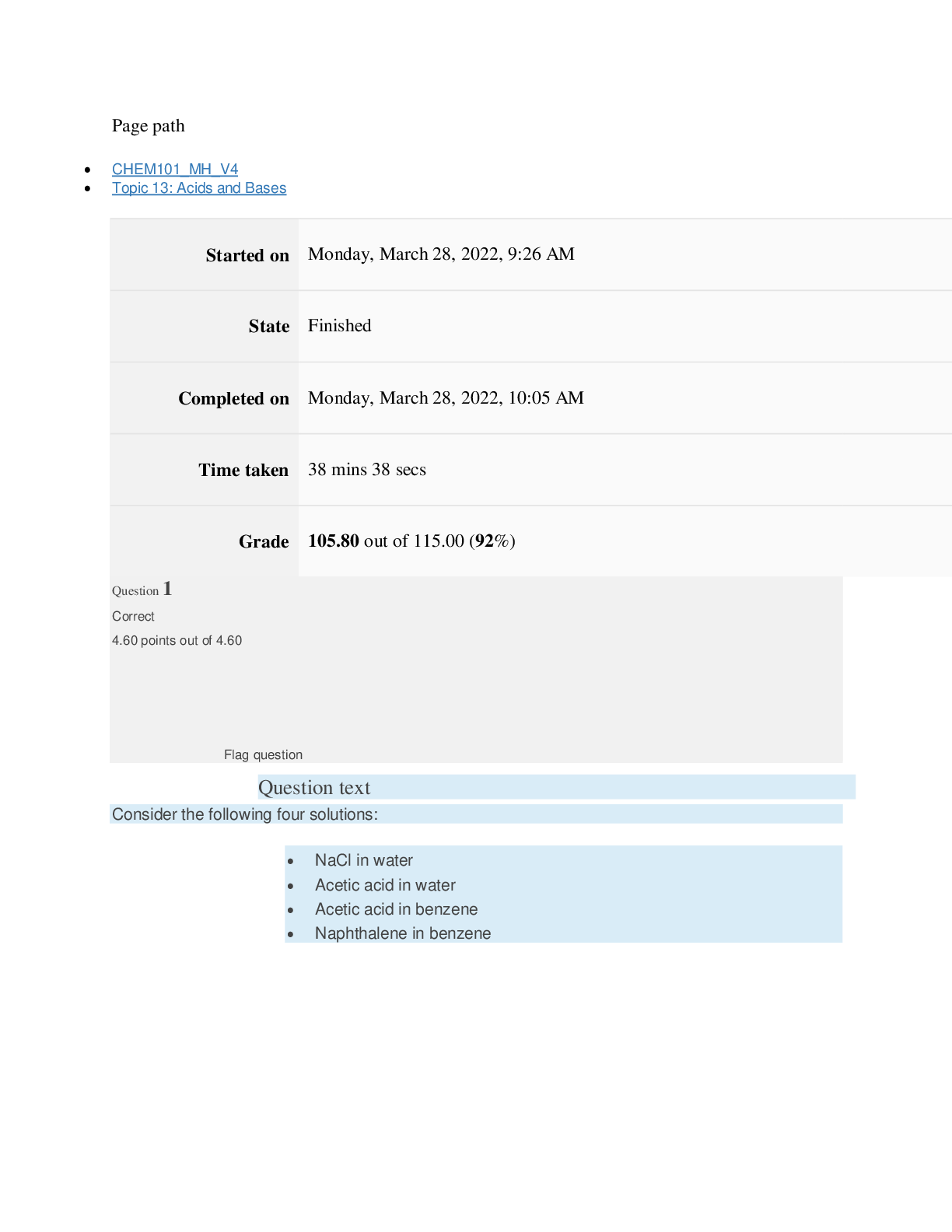
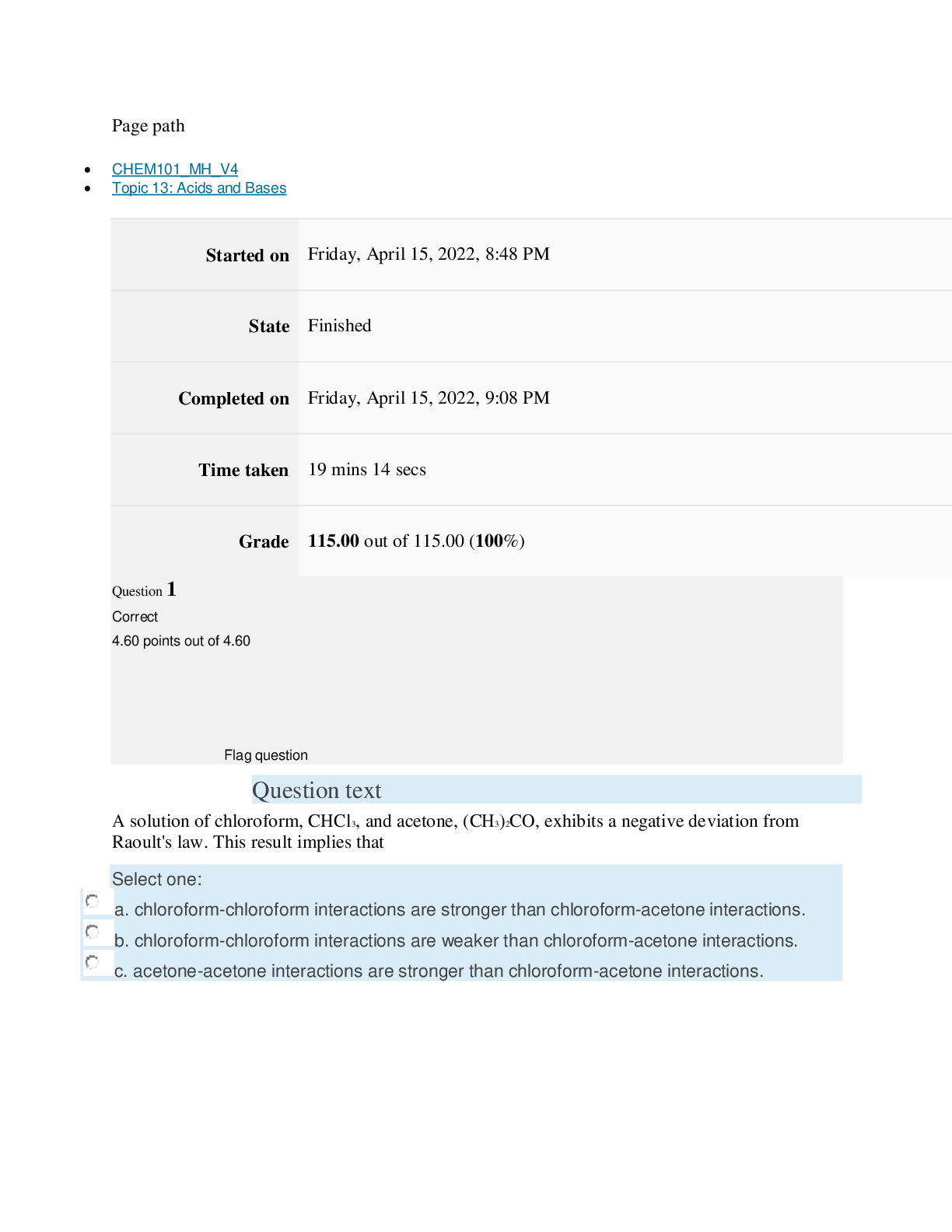
Page path CHEM101_MH_V4 Topic 13: Acids and Bases Started on Friday, April 15, 2022, 8:48 PM State Finished Completed on Friday, April 15, 2022, 9:08 PM Time taken 19 mins 14 secs Grade 115... .00 out of 115.00 (100%) Question 1 Correct 4.60 points out of 4.60 Flag question Question text A solution of chloroform, CHCl3, and acetone, (CH3)2CO, exhibits a negative deviation from Raoult's law. This result implies that Select one: a. chloroform-chloroform interactions are stronger than chloroform-acetone interactions. b. chloroform-chloroform interactions are weaker than chloroform-acetone interactions. c. acetone-acetone interactions are stronger than chloroform-acetone interactions. Question text At 10°C one volume of water dissolves 3.10 volumes of chlorine gas at 1.00 atm pressure. What is the Henry's law constant of Cl2 in water? (R = 0.0821 atm • L • mol–1• K–1 ) Question text What is the vapor pressure above a solution prepared by dissolving 0.500 mol of a nonvolatile solute in 275 g of hexane (86.18 g/mol) at 49.6°C? P°hexane = 400.0 torr at 49.6°C. Question 3 Correct 4.60 points out of 4.60 Flag question Select one: a. 3.8 mol • L–1• atm–1 b. 0.043 mol • L–1• atm–1 c. 36 mol • L–1• atm–1 d. 3.1 mol • L–1• atm–1 e. 0.13 mol • L–1• atm–1 e. Choice 2 and 4 d. acetone-acetone interactions are weaker than chloroform-acetone interactions. Flag question Question 2 Correct 4.60 points out of 4.60 Select one: a. 54 torr b. 154 torr c. 246 torr Question text A solution is 40.00% by volume benzene (C6H6) in carbon tetrachloride at 20°C. The vapor pressure of pure benzene at this temperature is 74.61 mmHg and its density is 0.87865 g/cm3; the vapor pressure of pure carbon tetrachloride is 91.32 mmHg and its density is 1.5940 g/cm3. If this solution is ideal, its total vapor pressure at 20°C is Question text Safrole was once used as a flavoring in root beer, until it was banned in 1960. What is the vapor pressure of a solution prepared by dissolving 0.75 mol of nonvolatile safrole in 950 g of ethanol (46.07 g/mol)? P°ethanol = 50.0 torr at 25°C. Flag question Question 5 Correct 4.60 points out of 4.60 e. 400. torr d. 346 torr Flag question Question 4 Correct 4.60 points out of 4.60 c. 82.96 mmHg d. 81.63 mmHg e. 165.93 mmHg b. 84.30 mmHg Select one: a. 84.64 mmHg Select one: a. 1.8 torr b. 11 torr [Show More]
Last updated: 1 year ago
Preview 1 out of 14 pages
Instant download
Buy this document to get the full access instantly
Instant Download Access after purchase
Add to cartInstant download
Reviews( 0 )
Document information
Connected school, study & course
About the document
Uploaded On
Dec 06, 2022
Number of pages
14
Written in
Additional information
This document has been written for:
Uploaded
Dec 06, 2022
Downloads
0
Views
60