Chemistry > Lab Experiment > University of WashingtonCHEM 162-lab 3-ACCURATE RESULTS AS VERIFIED BY EXPERTS (All)
University of WashingtonCHEM 162-lab 3-ACCURATE RESULTS AS VERIFIED BY EXPERTS
Document Content and Description Below
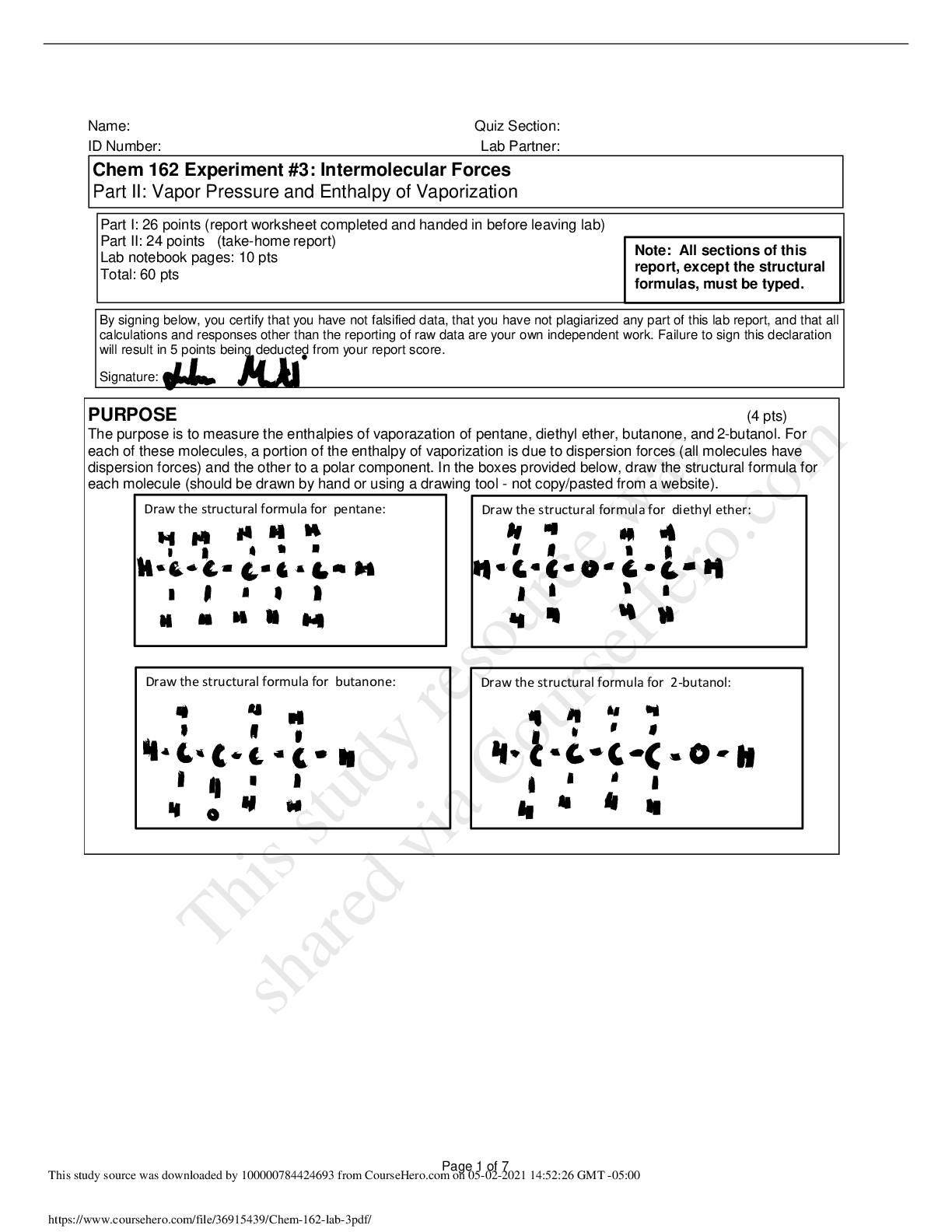
Chem 162 Experiment #3: Intermolecular Forces Part II: Vapor Pressure and Enthalpy of Vaporization PURPOSE (4 pts) The purpose is to measure the enthalpies of vaporazation of pentane, diethyl ether... , butanone, and 2-butanol. For each of these molecules, a portion of the enthalpy of vaporization is due to dispersion forces (all molecules have dispersion forces) and the other to a polar component. In the boxes provided below, draw the structural formula for each molecule (should be drawn by hand or using a drawing tool - not copy/pasted from a website). Part I: 26 points (report worksheet completed and handed in before leaving lab) Part II: 24 points (take-home report) Lab notebook pages: 10 pts Total: 60 pts By signing below, you certify that you have not falsified data, that you have not plagiarized any part of this lab report, and that all calculations and responses other than the reporting of raw data are your own independent work. Failure to sign this declaration will result in 5 points being deducted from your report score. Signature: Draw the structural formula for pentane: Draw the structural formula for diethyl ether: Draw the structural formula for butanone: Draw the structural formula for 2-butanol: Note: All sections of this report, except the structural formulas, must be typed. Page 1 of 7 DATA, CALCULATIONS AND GRAPHS Pentane Temperature (oC) Temperature (K) 1/Temp (1/K) Pvap (mmHg) Pvap (atm) ln Pvap 11.8 285.0 0.003509 341.80 0.44974 -0.7991 14.9 288.1 0.003472 361.50 0.47566 -0.7431 17.7 290.9 0.003438 381.50 0.50197 -0.6892 19.6 292.8 0.003416 401.50 0.52829 -0.6381 21.6 294.8 0.003393 421.50 0.55461 -0.5895 23.2 296.4 0.003374 441.50 0.58092 -0.5431 24.8 298.0 0.003356 461.50 0.60724 -0.4988 26.2 299.4 0.003341 481.50 0.63355 -0.4564 27.8 301.0 0.003323 501.50 0.65987 -0.4157 28.9 302.1 0.003311 521.50 0.68618 -0.3766 30.4 303.6 0.003294 541.50 0.71250 -0.3390 31.3 304.5 0.003285 561.50 0.73882 -0.3027 32.6 305.8 0.003271 581.50 0.76513 -0.2677 33.6 306.8 0.003260 601.50 0.79145 -0.2339 34.6 307.8 0.003249 621.50 0.81776 -0.2012 35.4 308.6 0.003241 641.50 0.84408 -0.1695 36.4 309.6 0.003230 661.50 0.87039 -0.1388 37.4 310.6 0.003220 681.50 0.89671 -0.1090 38.3 311.5 0.003211 701.50 0.92303 -0.0801 39.1 312.3 0.003203 721.50 0.94934 -0.0520 4 pts 2 pts DHo vap 21.09 kJ/mol DSo vap 66.84 J/(mol·K) Type sample calculations for determining DH and DS for n-pentane. Slope = -2536.5 Delta H = -Slope x R = -(2536.5) x 8.3145 J/molK x 1kJ/1000J = 21.0897kJ/mol Intercept = 8.0387 Delta S = Intercept x R = 8.0387 x 8.3145 J/molK = 66.8378J/molK .Place your plot of ln(Pvap) vs 1/T here (cover this instruction box so your graph is an appropriate size) .Properly label your graph (labels for axes, including units, and a title) Include a trendline and its equation and R2 value on your graph. This is done by right-clicking on one of the data points on your graph and choosing "Add Trendline" from the drop down menu. The first tab asks what type of trendline you .wish to use, and the Options tab allows you to include the trendline equation and R2 value y = -2536.5x + 8.0387 R² = 0.9889 -1.0000 -0.9000 -0.8000 -0.7000 -0.6000 -0.5000 -0.4000 -0.3000 -0.2000 -0.1000 0.0000 0.003150 0.003200 0.003250 0.003300 0.003350 0.003400 0.003450 0.003500 0.003550 lnPvap 1/Temp (1/K) Pentane: lnPvap vs. 1/T Page 2 of 7 [Show More]
Last updated: 1 year ago
Preview 1 out of 7 pages
Instant download
Buy this document to get the full access instantly
Instant Download Access after purchase
Add to cartInstant download
Reviews( 0 )
Document information
Connected school, study & course
About the document
Uploaded On
May 02, 2021
Number of pages
7
Written in
Additional information
This document has been written for:
Uploaded
May 02, 2021
Downloads
0
Views
73














