Boyle’s Law and Charles’s Law SE Answer key
Document Content and Description Below
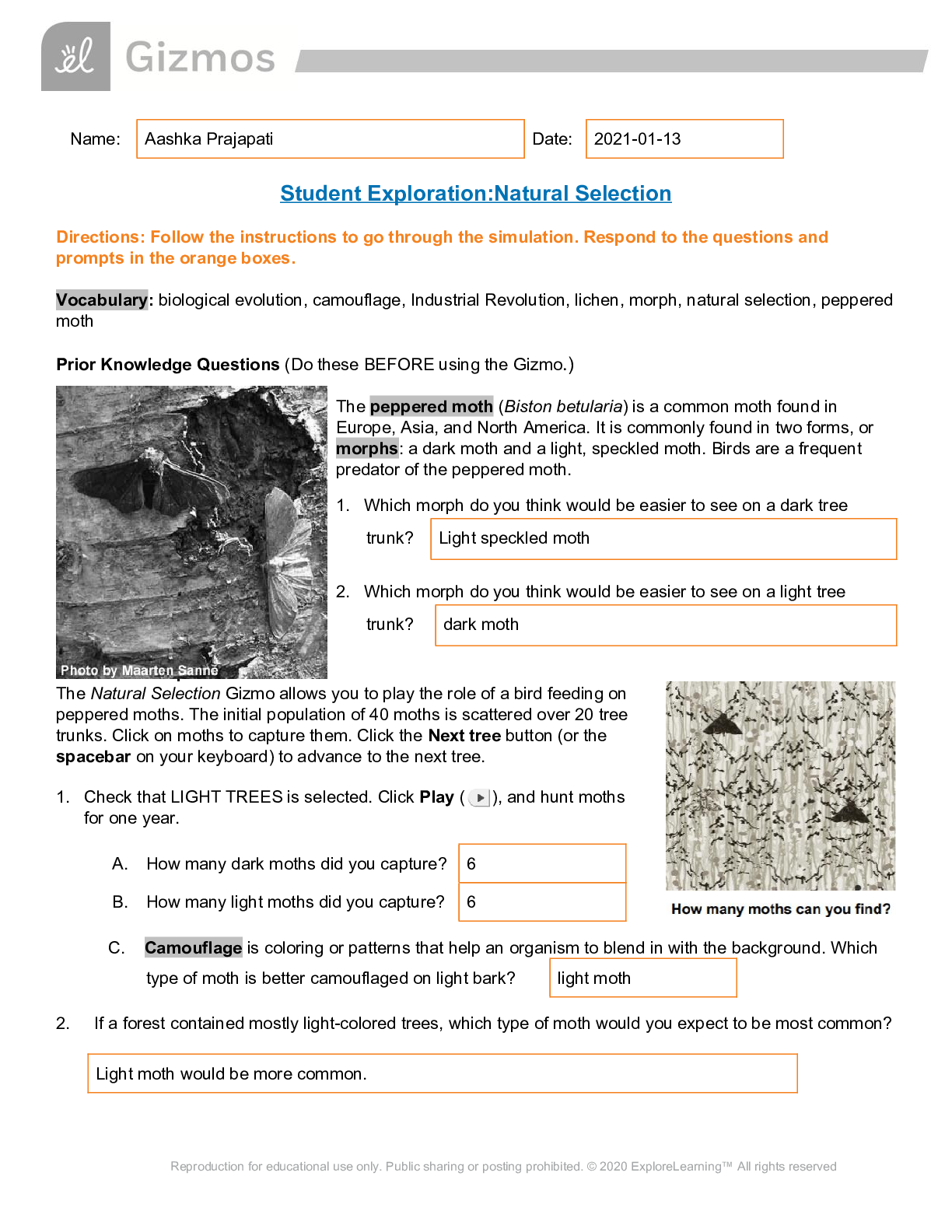
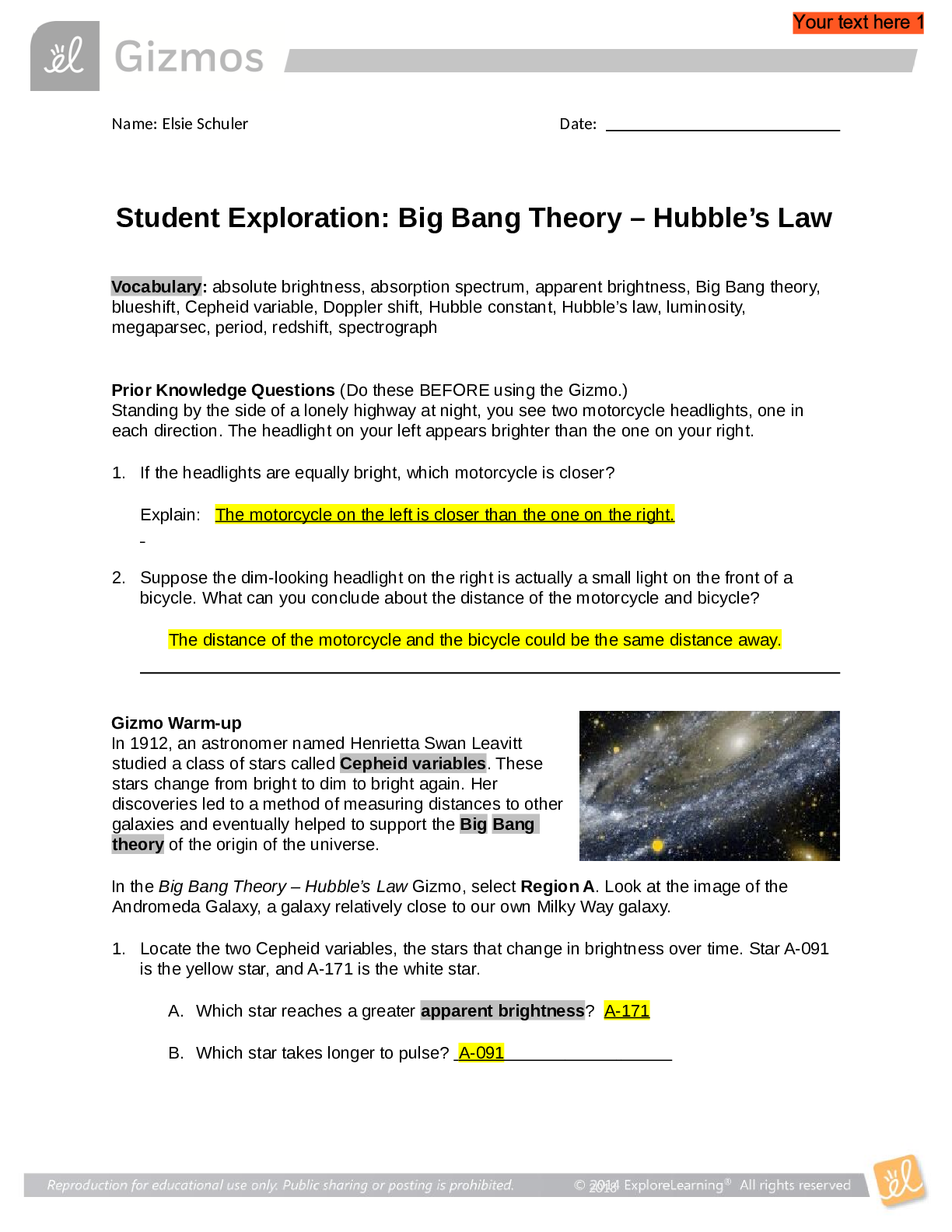
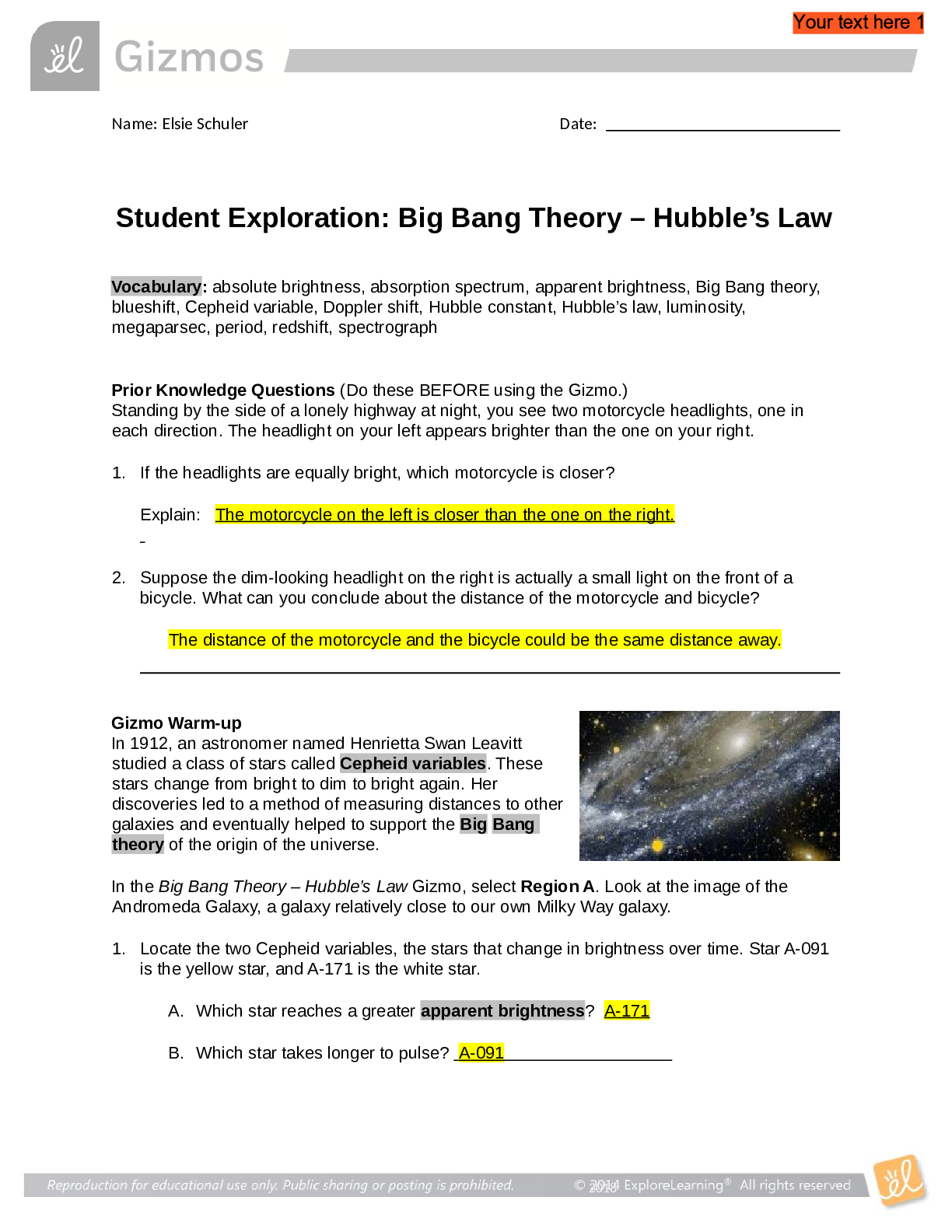
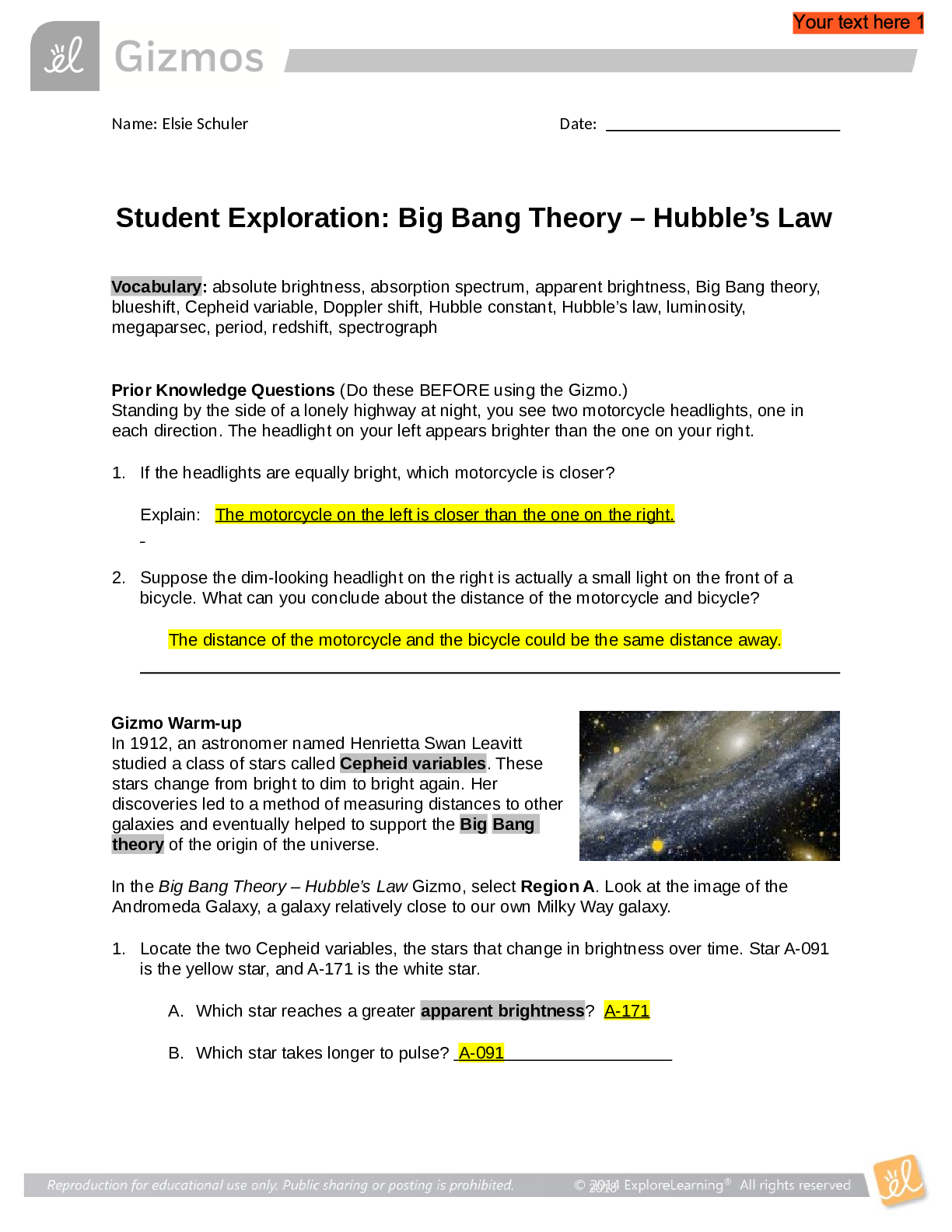
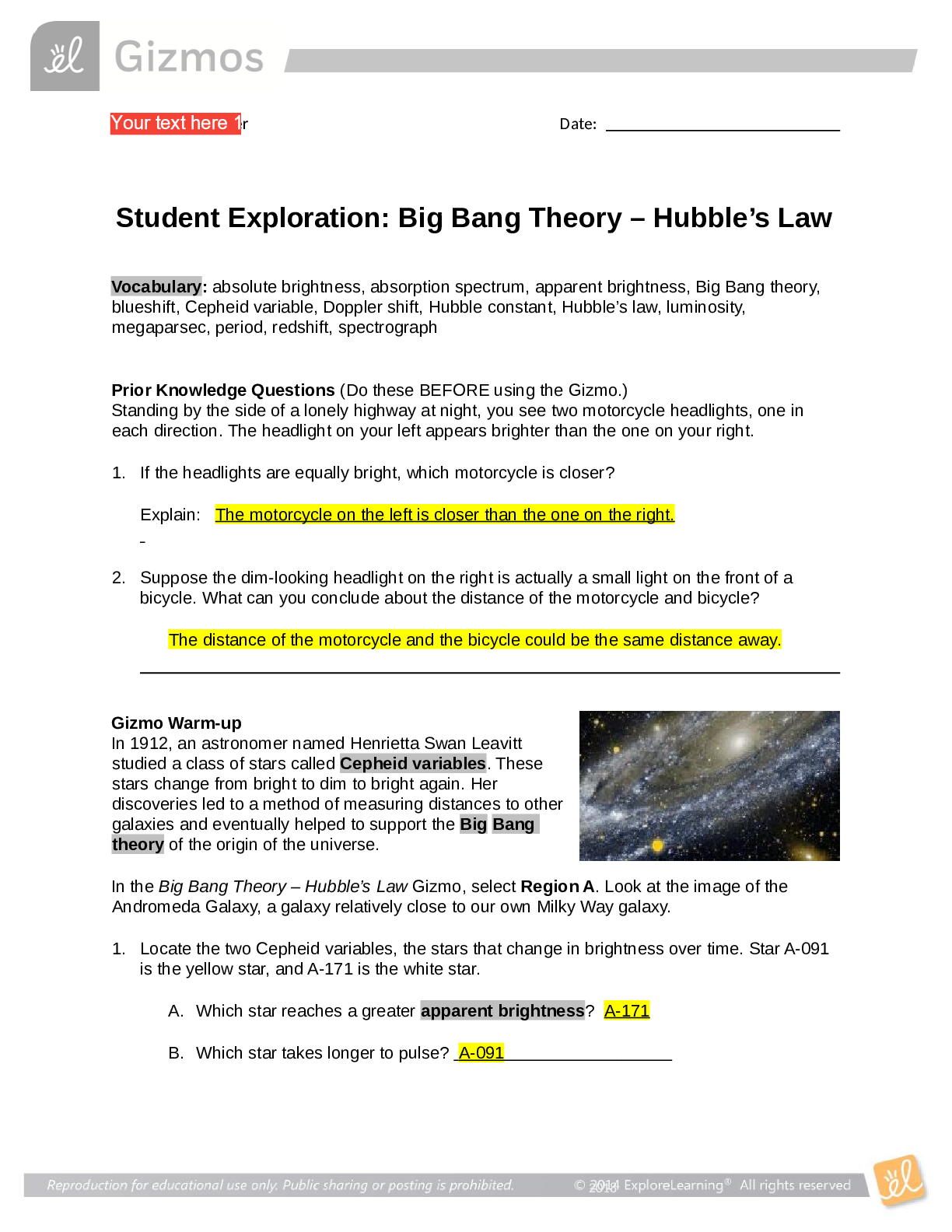
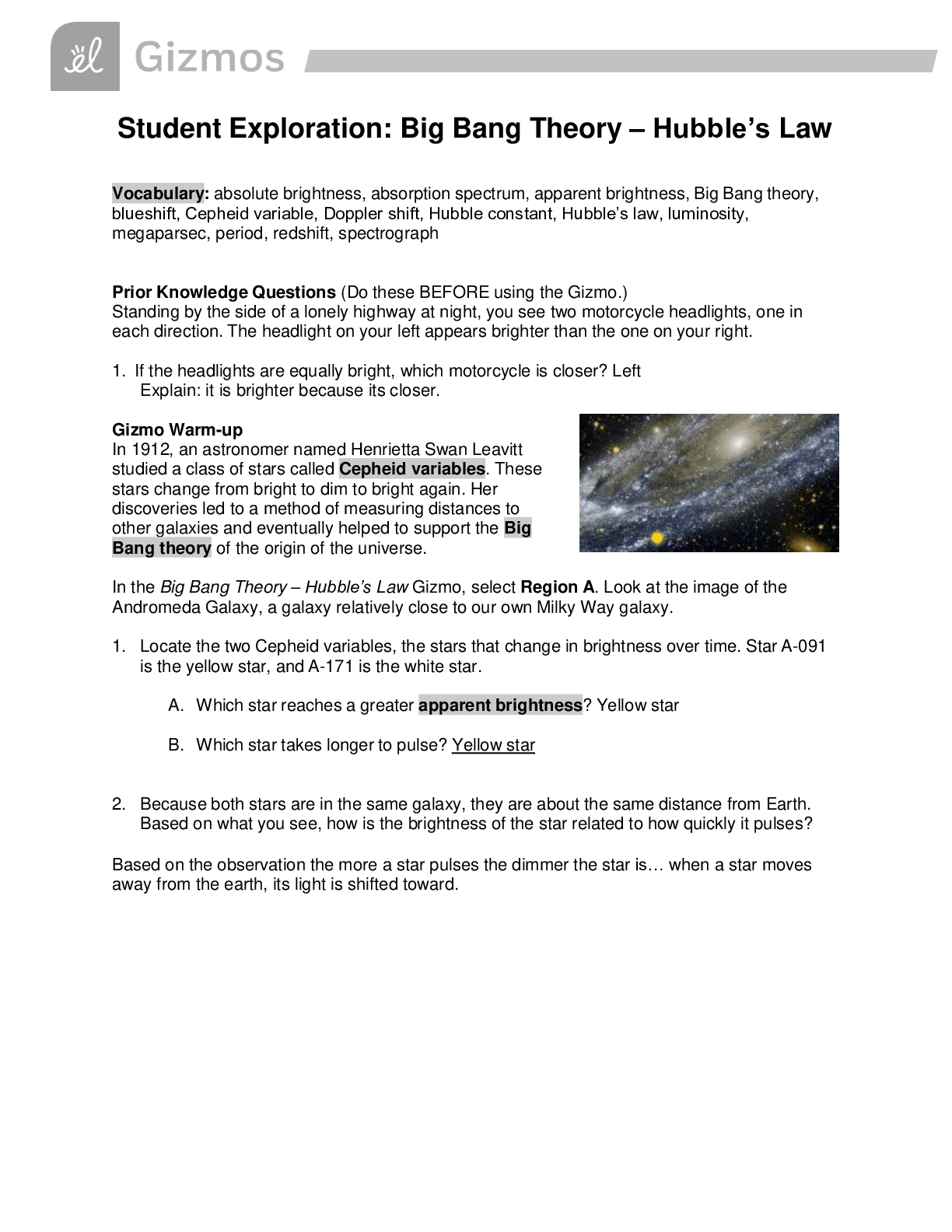
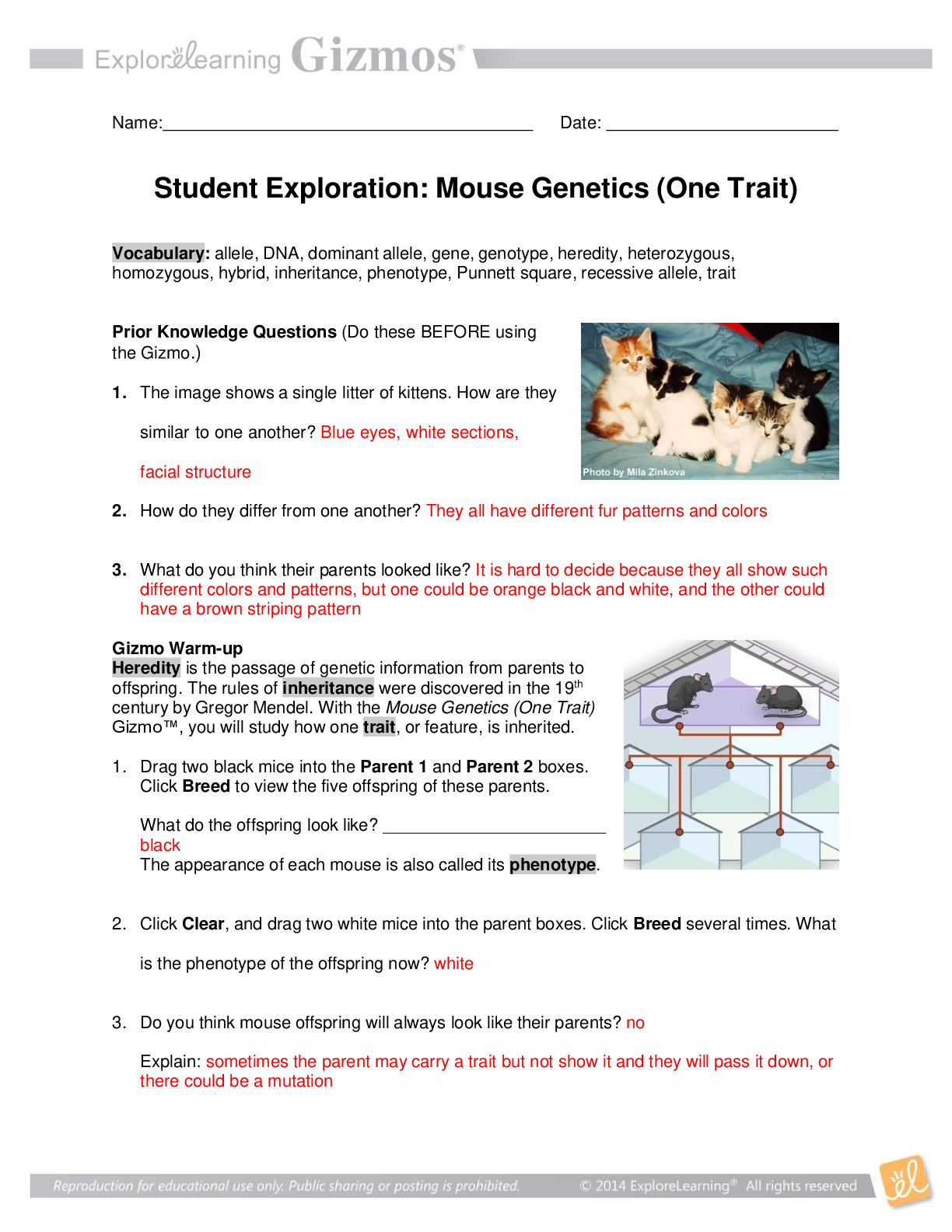
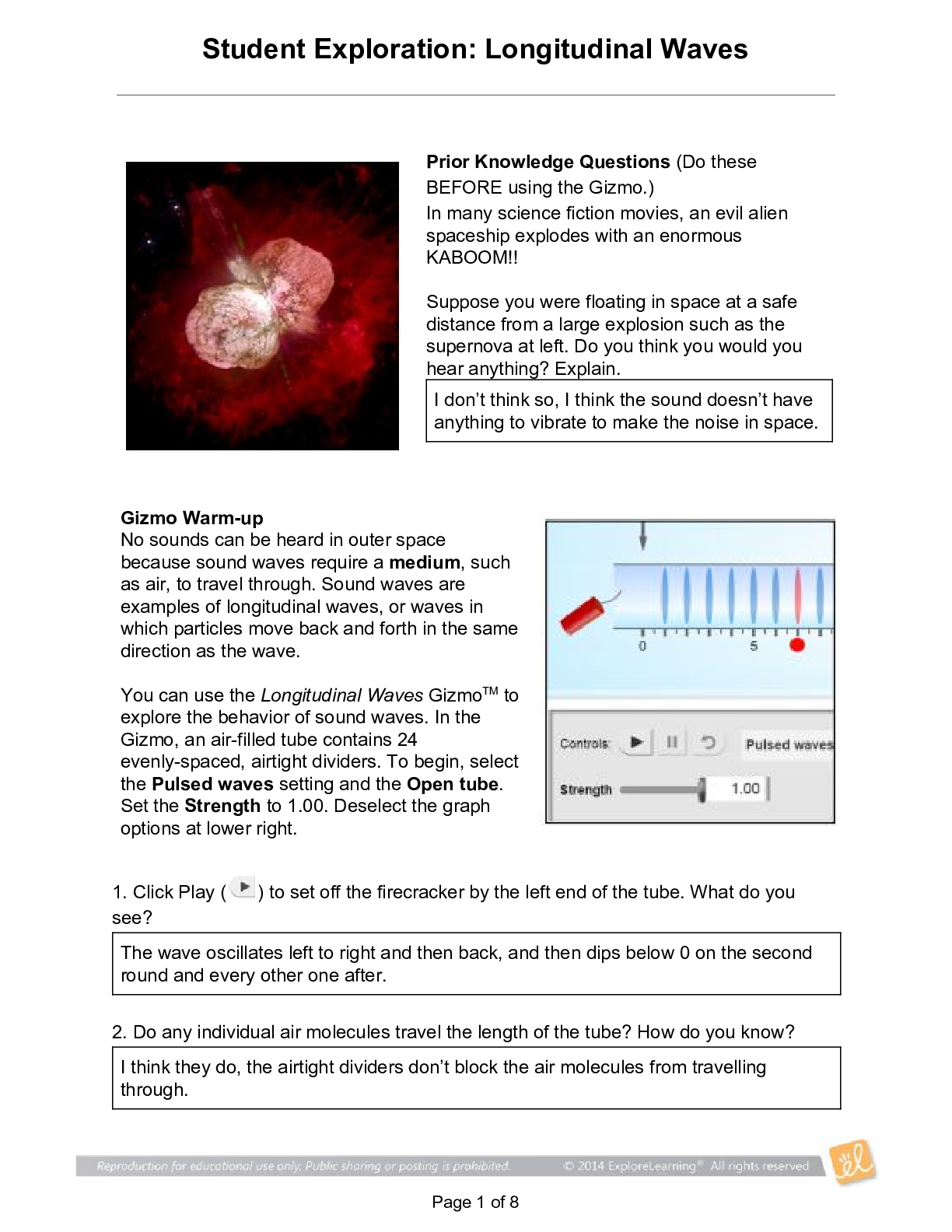
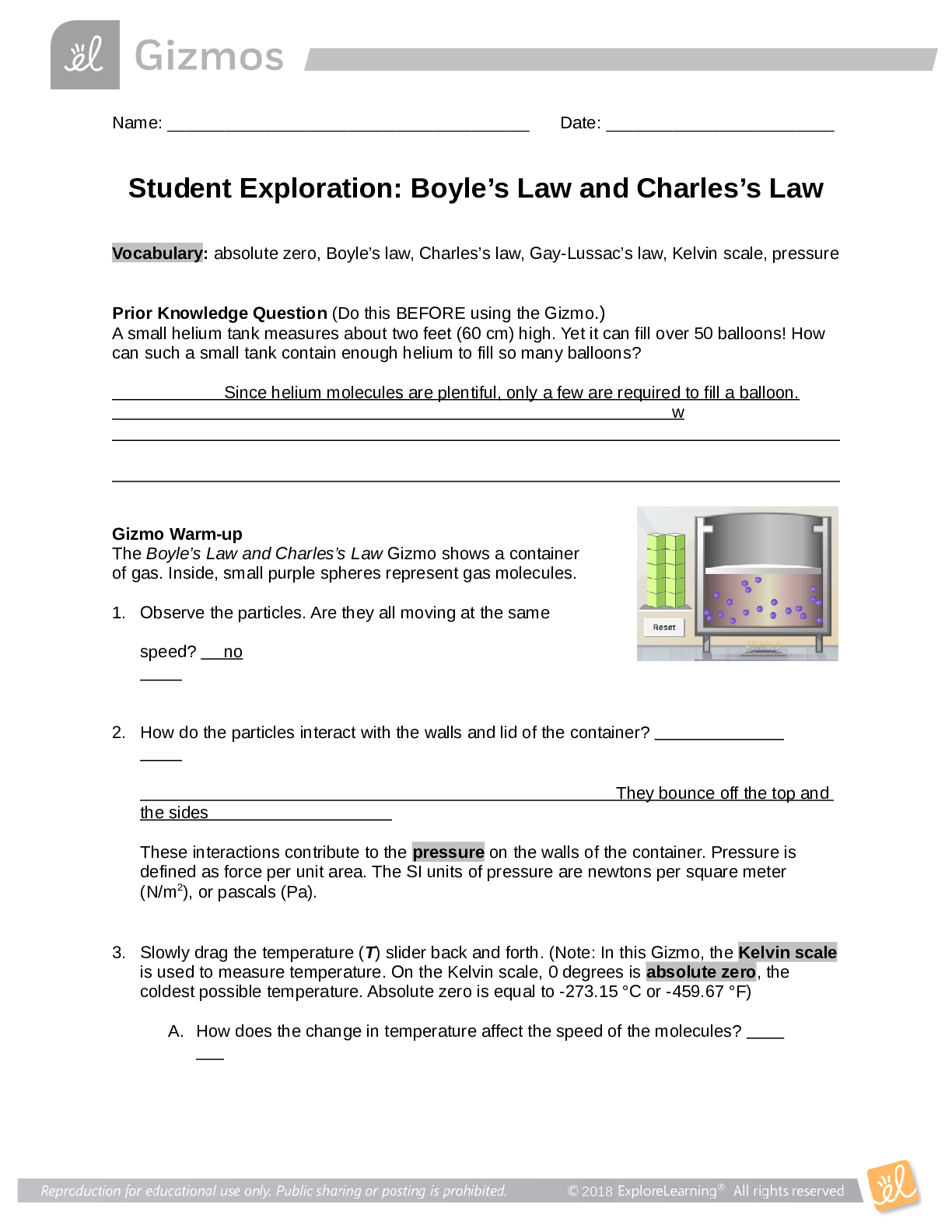
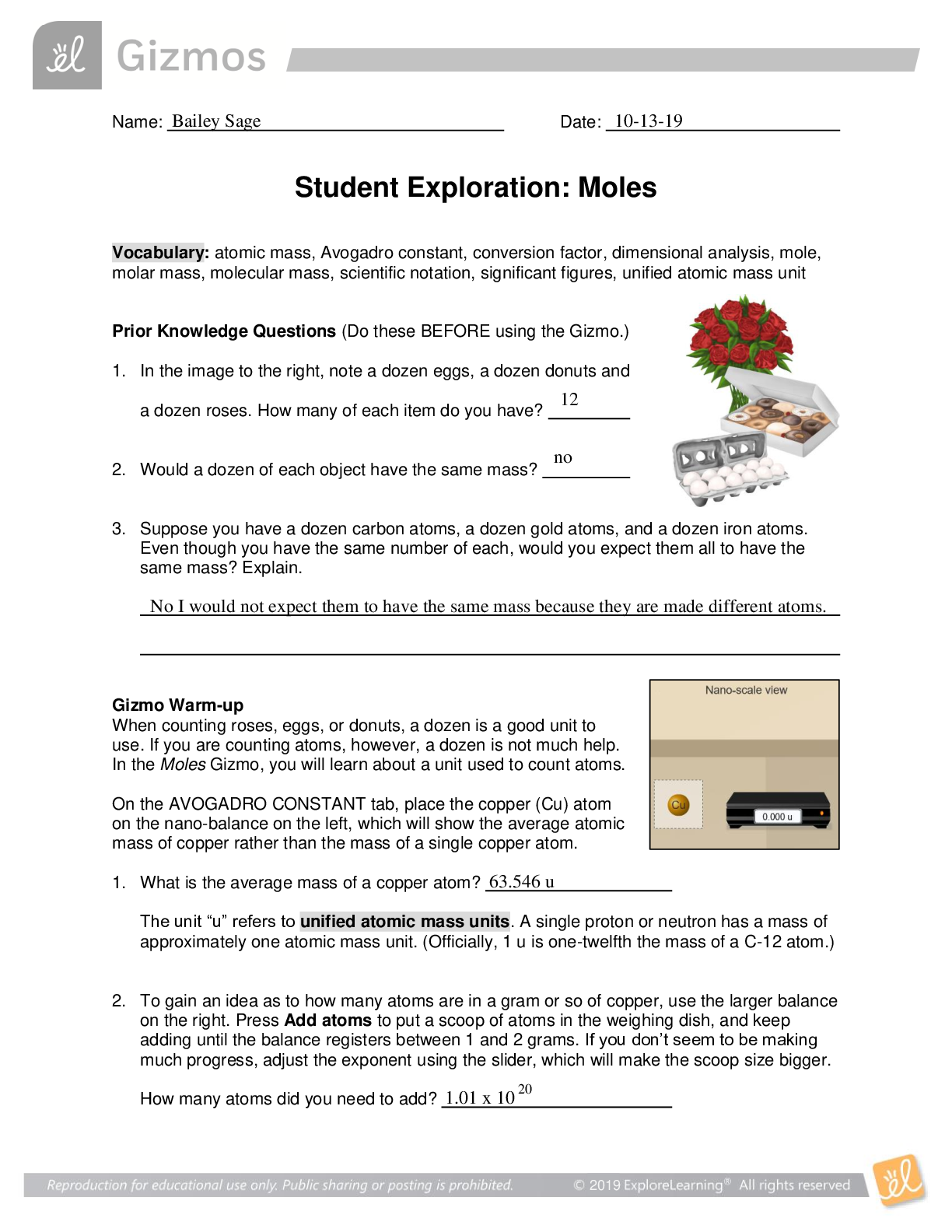
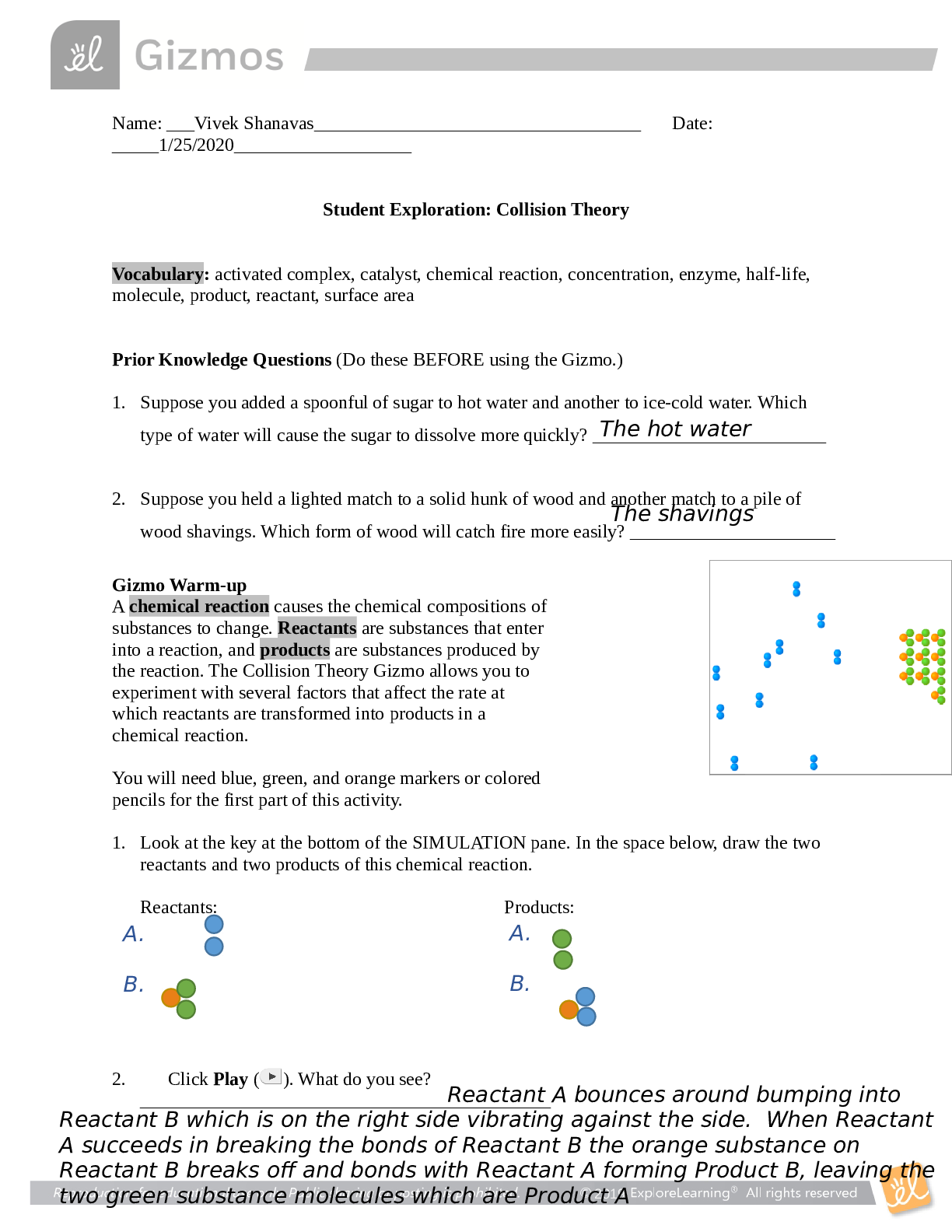
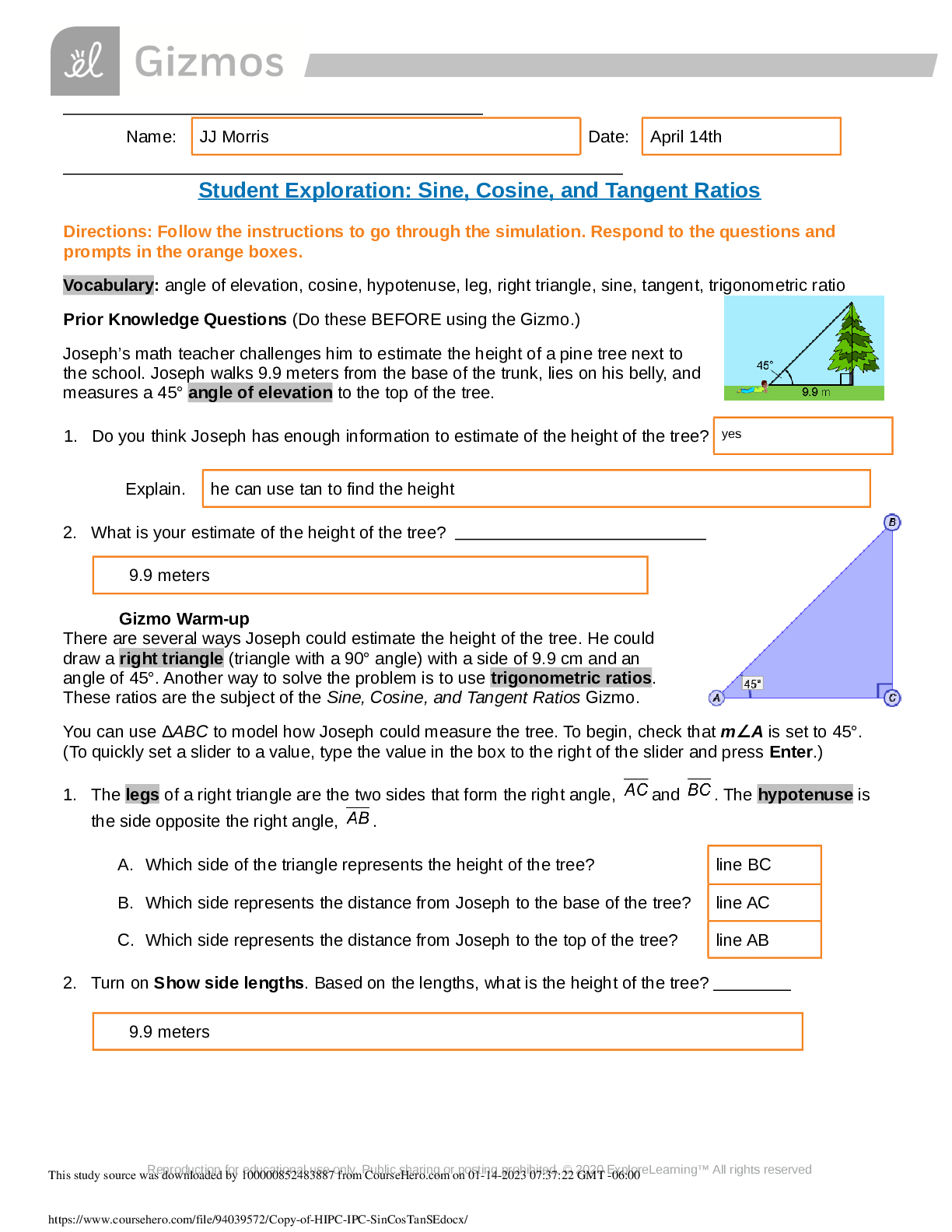
Student Exploration: Boyle’s Law and Charles’s Law Vocabulary: absolute zero, Boyle’s law, Charles’s law, Gay-Lussac’s law, Kelvin scale, pressure Prior Knowledge Question (Do this BEFORE ... using the Gizmo.) A small helium tank measures about two feet (60 cm) high. Yet it can fill over 50 balloons! How can such a small tank contain enough helium to fill so many balloons? Since helium molecules are plentiful, only a few are required to fill a balloon. w Gizmo Warm-up The Boyle’s Law and Charles’s Law Gizmo shows a container of gas. Inside, small purple spheres represent gas molecules. 1. Observe the particles. Are they all moving at the same speed? no 2. How do the particles interact with the walls and lid of the container? They bounce off the top and the sides These interactions contribute to the pressure on the walls of the container. Pressure is defined as force per unit area. The SI units of pressure are newtons per square meter (N/m2 ), or pascals (Pa). 3. Slowly drag the temperature (T) slider back and forth. (Note: In this Gizmo, the Kelvin scale is used to measure temperature. On the Kelvin scale, 0 degrees is absolute zero, the coldest possible temperature. Absolute zero is equal to -273.15 °C or -459.67 °F) A. How does the change in temperature affect the speed of the molecules? 2018 The pressure of the molecules are affected B. How does the change in temperature affect the volume of the container? How `````large or small the volume gets 2018 Activity A: Boyle’s law Get the Gizmo ready: Set the temperature (T) to 300 K. Check that the mass (m) is set to 0 kg. Question: How does pressure affect the volume of a gas? 1. Form hypothesis: In this experiment, you will pile weights on the lid of the container of gas. What do you think will happen as more weight is added to the lid? It increases the weight on the molecules and lowers the lid. 2. Notice: Look at the DESCRIPTION pane. What is the mass of the lid? 10kg How much pressure does the lid exert on the gas? 98.1 n/m2 3. Collect data: With the temperature held constant at 300 K, use the Select mass slider to place weights on the lid. Record the pressure and volume of the gas for each added mass. Added mass on the lid Total mass (lid + added mass) Pressure* Volume 0 kg 10 kg 98.1n/m2 2.54m3 10 kg 20 kg 196.n/m2 1.27m3 20 kg 30 kg 294.3n/m2 .85m3 30 kg 40 kg *This model does not include atmospheric pressure, which is 101,325 N/m2 . 4. Analyze: As the pressure increases at constant temperature, what happens to the volume of the gas? when the pressure of a gas at constant temperature is increased, the volume of the gas decreases. when the pressure is decreased, the volume increases This relationship is called Boyle’s law. [Show More]
Last updated: 1 year ago
Preview 1 out of 14 pages
Also available in bundle (1)

(BUNDLE) Gizmos | STEM Simulations & Virtual Labs -ANSWER KEYS
(BUNDLE) Gizmos | STEM Simulations & Virtual Labs -ANSWER KEYS
By Academia1434 4 months ago
$90
57
Reviews( 0 )
Document information
Connected school, study & course
About the document
Uploaded On
May 13, 2021
Number of pages
14
Written in
Additional information
This document has been written for:
Uploaded
May 13, 2021
Downloads
0
Views
101


 Answer key (TOP RATED).png)








, Questions and Answers, All Correct Study Guide, Download to Score A.png)