Chemistry > Study Notes > Revision Notes - Unit 1 AQA Chemistry A-level. (All)
Revision Notes - Unit 1 AQA Chemistry A-level.
Document Content and Description Below
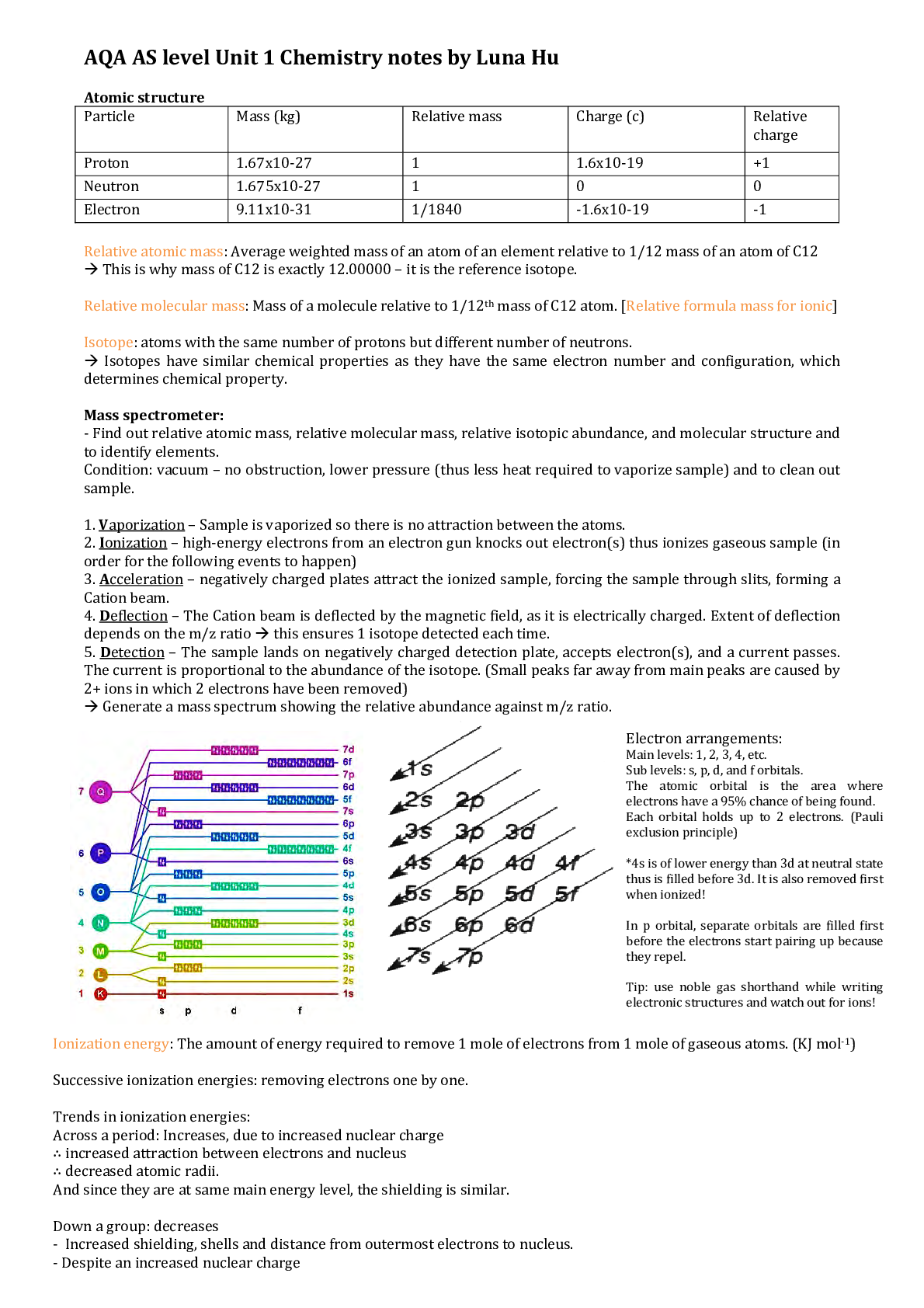
AQA AS level Unit 1 Chemistry notes by Luna Hu Atomic structure Particle Mass (kg) Relative mass Charge (c) Relative charge Proton 1.67x10-27 1 1.6x10-19 +1 Neutron 1.675x10-27 1 0 0 Electron 9.... 11x10-31 1/1840 -1.6x10-19 -1 Relative atomic mass: Average weighted mass of an atom of an element relative to 1/12 mass of an atom of C12 This is why mass of C12 is exactly 12.00000 – it is the reference isotope. Relative molecular mass: Mass of a molecule relative to 1/12th mass of C12 atom. [Relative formula mass for ionic] Isotope: atoms with the same number of protons but different number of neutrons. Isotopes have similar chemical properties as they have the same electron number and configuration, which determines chemical property. Mass spectrometer: - Find out relative atomic mass, relative molecular mass, relative isotopic abundance, and molecular structure and to identify elements. Condition: vacuum – no obstruction, lower pressure (thus less heat required to vaporize sample) and to clean out sample. 1. Vaporization – Sample is vaporized so there is no attraction between the atoms. 2. Ionization – high-energy electrons from an electron gun knocks out electron(s) thus ionizes gaseous sample (in order for the following events to happen) 3. Acceleration – negatively charged plates attract the ionized sample, forcing the sample through slits, forming a Cation beam. 4. Deflection – The Cation beam is deflected by the magnetic field, as it is electrically charged. Extent of deflection depends on the m/z ratio this ensures 1 isotope detected each time. 5. Detection – The sample lands on negatively charged detection plate, accepts electron(s), and a current passes. The current is proportional to the abundance of the isotope. (Small peaks far away from main peaks are caused by 2+ ions in which 2 electrons have been removed) Generate a mass spectrum showing the relative abundance against m/z ratio [Show More]
Last updated: 1 year ago
Preview 1 out of 6 pages
Reviews( 0 )
Document information
Connected school, study & course
About the document
Uploaded On
Jul 06, 2021
Number of pages
6
Written in
Additional information
This document has been written for:
Uploaded
Jul 06, 2021
Downloads
0
Views
101

















.png)



.png)



How Do Geographically Dispersed Teams Collaborate Effectively Paper.png)








