CHEM 120 WEEK 8 FINAL EXAM
Document Content and Description Below
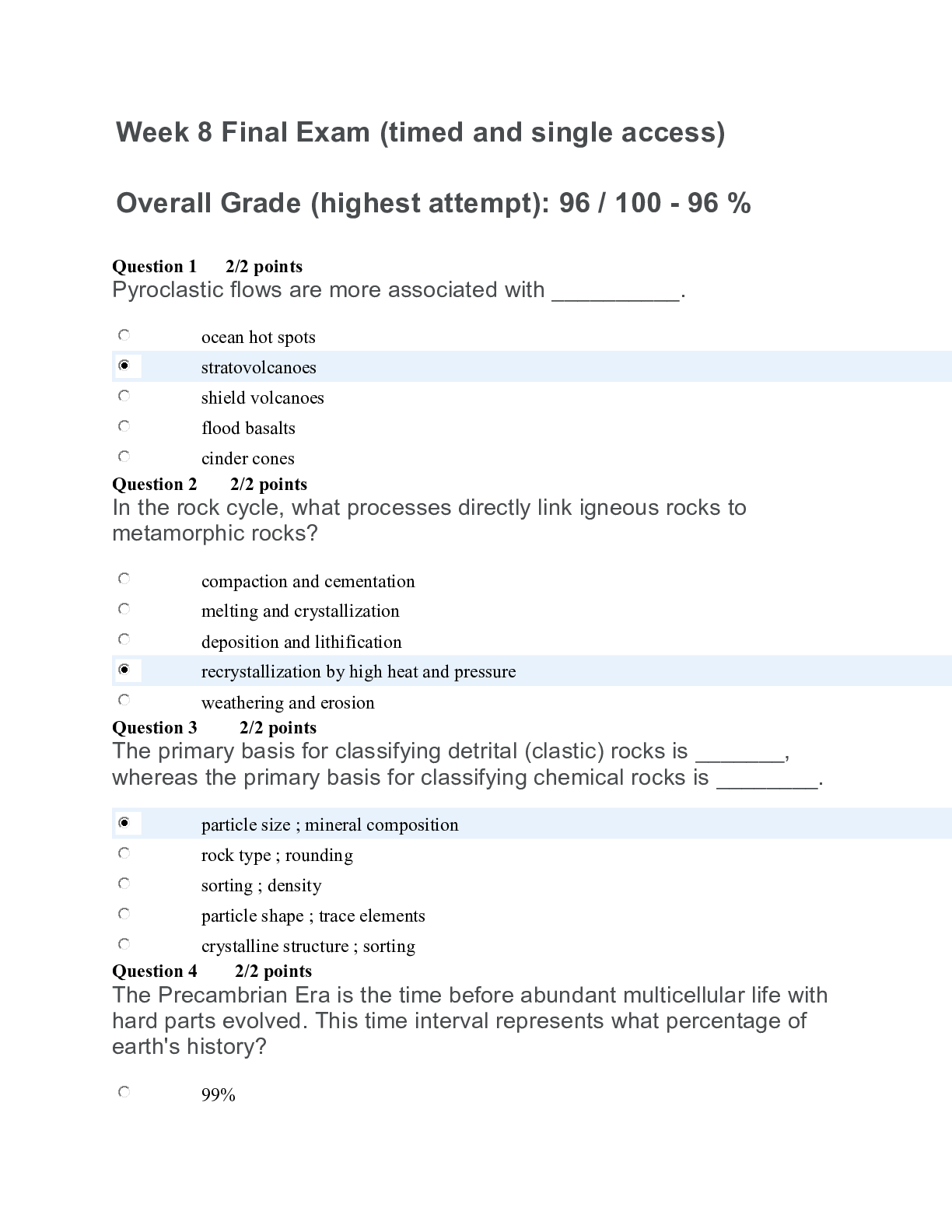
CHEM 120 WEEK 8 FINAL EXAM (TCO 8) 35.0 mL of 0.25 M NaOH is neutralized by 23.6 mL of an HCl solution. The molarity of the HCl solution is (show your work): (TCO 7) (a, 5 pts) Given that the molar... mass of H3PO4 is 97.994 grams, determine the number of grams of H3PO4 needed to prepare 0.75L of a 0.25M H3PO4 solution. Show your work. (b, 5 pts) What volume, in Liters, of a 0.25 M H3PO4 solution can be prepared by diluting 50 mL of a 2.5M H3PO4 solution? [Show More]
Last updated: 1 year ago
Preview 1 out of 20 pages

Reviews( 0 )
Document information
Connected school, study & course
About the document
Uploaded On
Mar 22, 2023
Number of pages
20
Written in
Additional information
This document has been written for:
Uploaded
Mar 22, 2023
Downloads
0
Views
39







.png)




















.png)