*NURSING > Study Notes > NURS N4581 Exam 2 Week 8 Structural, Infectious, and Inflammatory Cardiovascular Disorders | Downloa (All)
NURS N4581 Exam 2 Week 8 Structural, Infectious, and Inflammatory Cardiovascular Disorders | Download To Score An A
Document Content and Description Below
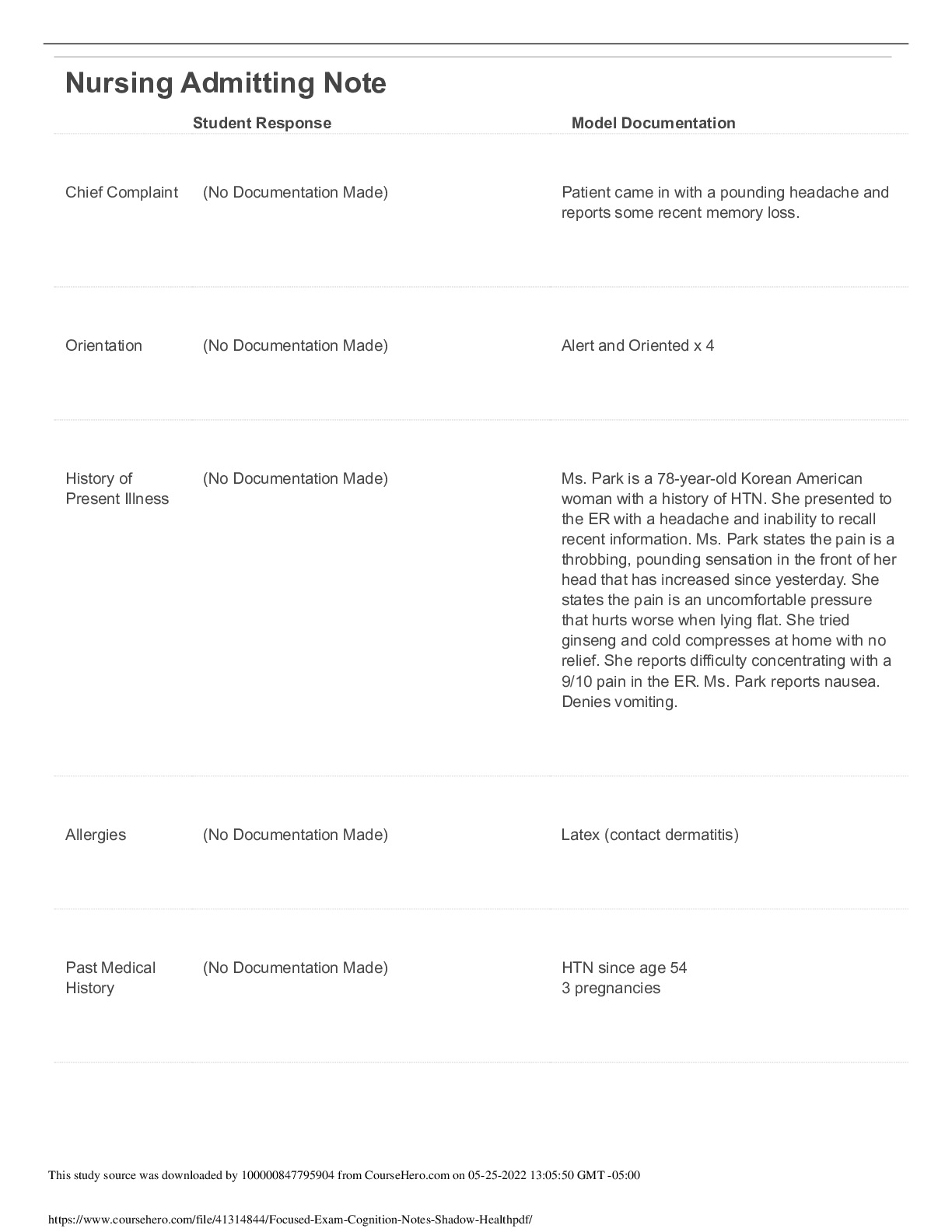
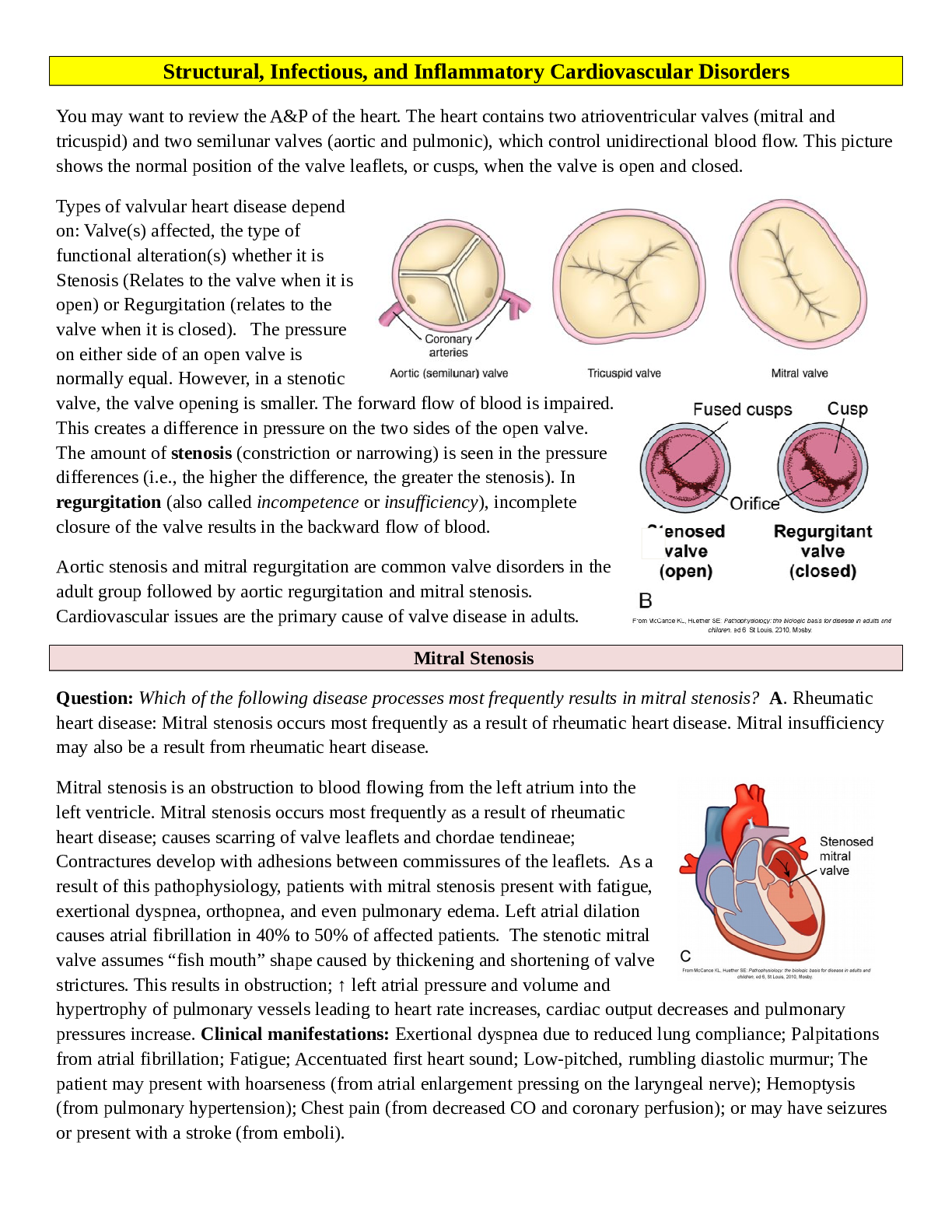
You may want to review the A&P of the heart. The heart contains two atrioventricular valves (mitral and tricuspid) and two semilunar valves (aortic and pulmonic), which control unidirectional blood fl... ow. This picture shows the normal position of the valve leaflets, or cusps, when the valve is open and closed. Types of valvular heart disease depend on: Valve(s) affected, the type of functional alteration(s) whether it is Stenosis (Relates to the valve when it is open) or Regurgitation (relates to the valve when it is closed). The pressure on either side of an open valve is normally equal. However, in a stenotic valve, the valve opening is smaller. The forward flow of blood is impaired. This creates a difference in pressure on the two sides of the open valve. The amount of stenosis (constriction or narrowing) is seen in the pressure differences (i.e., the higher the difference, the greater the stenosis). In regurgitation (also called incompetence or insufficiency), incomplete closure of the valve results in the backward flow of blood. Aortic stenosis and mitral regurgitation are common valve disorders in the adult group followed by aortic regurgitation and mitral stenosis. Cardiovascular issues are the primary cause of valve disease in adults. Question: Which of the following disease processes most frequently results in mitral stenosis? A. Rheumatic heart disease: Mitral stenosis occurs most frequently as a result of rheumatic heart disease. Mitral insufficiency may also be a result from rheumatic heart disease. Mitral stenosis is an obstruction to blood flowing from the left atrium into the left ventricle. Mitral stenosis occurs most frequently as a result of rheumatic heart disease; causes scarring of valve leaflets and chordae tendineae; Contractures develop with adhesions between commissures of the leaflets. As a result of this pathophysiology, patients with mitral stenosis present with fatigue, exertional dyspnea, orthopnea, and even pulmonary edema. Left atrial dilation causes atrial fibrillation in 40% to 50% of affected patients. The stenotic mitral valve assumes “fish mouth” shape caused by thickening and shortening of valve strictures. This results in obstruction; ↑ left atrial pressure and volume and hypertrophy of pulmonary vessels leading to heart rate increases, cardiac output decreases and pulmonary pressures increase. Clinical manifestations: Exertional dyspnea due to reduced lung compliance; Palpitations from atrial fibrillation; Fatigue; Accentuated first heart sound; Low-pitched, rumbling diastolic murmur; The patient may present with hoarseness (from atrial enlargement pressing on the laryngeal nerve); Hemoptysis (from pulmonary hypertension); Chest pain (from decreased CO and coronary perfusion); or may have seizures or present with a stroke (from emboli). Mitral insufficiency involves blood flowing back from the left ventricle into the left atrium during systole. Often, edges of mitral valve leaflets do not close completely during systole because leaflets and chordae tendineae have thickened and fibrosed, resulting in their contraction. The most common causes of mitral valve regurgitation in are degenerative changes of the mitral valve (e.g., mitral valve prolapse) and ischemia of the left ventricle. Mitral insufficiency can occur acutely or develop over a period of time. Chronic mitral insufficiency may result from rheumatic heart disease; degenerative changes associated with aging, or left ventricular dilation. Volume overload can be reflected backward to the pulmonary circulation; however, pulmonary and right-sided heart symptoms usually do not develop until late in the disease process. As a result of this pathophysiology, patients with chronic mitral insufficiency commonly present with fatigue, palpitations, and sometimes shortness of breath. Acute mitral insufficiency may result from endocarditis, chest trauma, or myocardial infarction. In acute mitral insufficiency, cardiac output decreases dramatically, cascading into pulmonary edema and shock. The treatment of choice for acute mitral regurgitation with hemodynamic compromise is emergent mitral valve replacement. Question: The nurse is caring for a patient with aortic stenosis. For what should the nurse assess the patient? A Clinical manifestations of aortic stenosis include angina, syncope, dyspnea on exertion, heart failure, normal or soft S1, diminished or absent S2, systolic murmur, and prominent S4. Congenital aortic stenosis is generally found in childhood, adolescence, or young adulthood. In older adults, aortic stenosis (AS) is a result of Rheumatic Fever or degeneration; it occurs due to rheumatic heart disease, mitral valve disease will accompany it. Isolated AS is usually non-rheumatic in origin. The incidence of rheumatic aortic valve disease has been decreasing, but degenerative stenosis is increasing as the population ages. Aortic Stenosis causes obstruction of flow from the left ventricle to the aorta during systole. Aortic stenosis may develop as a result of rheumatic fever, calcification of a congenital bicuspid valve, or calcific degeneration, especially in elderly patients. Left ventricular hypertrophy and dilation results and increased left ventricular pressures are reflected backward through the left atrium and pulmonary vasculature. This increases myocardial oxygen consumption because of the increased myocardial mass. Diminished cardiac output in the person with aortic stenosis may lead to two major problems—angina and syncope. Patients with aortic stenosis also experience exertional dyspnea, orthopnea, and paroxysmal nocturnal dyspnea. As the disease progresses and compensatory mechanisms fail, reduced CO leads to decreased tissue perfusion, pulmonary hypertension, and HF. Symptoms of AS develop when the valve orifice becomes about one third its normal size. Symptoms include the classic triad of angina, syncope, and exertional dyspnea, reflecting left ventricular failure; auscultation of AS typically reveals a normal or soft S1, a diminished or absent S2, a systolic murmur, and a prominent S4. The prognosis is poor for a patient with symptoms and whose valve obstruction is not fixed. Nitroglycerin is used cautiously to treat angina as it can significantly reduce BP and worsen chest pain. Aortic regurgitation or insufficiency, like mitral insufficiency, can occur acutely or develop over a period of time. Chronic aortic insufficiency is commonly caused by rheumatic fever, reactive arthritis, and aneurysm of the ascending aorta. Left ventricular hypertrophy ensues. Eventually, the increase in left ventricular pressure is reflected backward into the left atrium and pulmonary circulation. Patients with chronic aortic insufficiency present with fatigue, and they have a low diastolic blood pressure and a widened pulse pressure and angina, exertional dyspnea, orthopnea, and paroxysmal nocturnal dyspnea Acute aortic insufficiency may be caused by blunt chest trauma, ruptured ascending aortic aneurysm or infective endocarditis and constitutes a life-threatening emergency. Physiologic consequence includes retrograde blood flow from ascending aorta back into left ventricle which results in volume overload. Initially, left ventricle compensates by dilation and hypertrophy but myocardial contractility eventually declines leading to pulmonary hypertension and right ventricular failure. Clinical manifestations are those of a sudden cardiovascular collapse: Left ventricle is exposed to aortic pressure during diastole resulting in sudden weakness; severe dyspnea; angina; and hypotension. In severe aortic regurgitation you can feel a “Water- hammer” pulse (a strong quick beat that collapses immediately). You may hear a soft or absent S1; presence of S3 and S4; and a soft, high-pitched diastolic murmur. History is always important with any patient. Have they had rheumatic fever or a murmur in the past? A CT scan of the chest with contrast is the gold standard for evaluating aortic disorders. An echocardiogram reveals valve structure, function, and heart chamber size. Transesophageal echocardiography reveals valve structure and function, and heart chamber size. Doppler color-flow imaging helps to diagnose and monitor valvular heart disease progression. Chest x-ray reveals the heart size, altered pulmonary circulation, and valve calcification. An ECG identifies heart rate, rhythm, and any ischemia or ventricular hypertrophy. Cardiac catheterization detects pressure changes in the cardiac chambers, records pressure differences across the valves, and measures the size of valve openings. An important aspect of conservative management of valvular heart disease is prevention of recurrent Respiratory Failure (RF) and Infective Endocarditis (IE); so prophylactic antibiotic therapy is used prior to any invasive procedure including dental visits. Treatment depends on the valve involved and disease severity. It focuses on preventing exacerbations of Heart Failure, acute pulmonary edema, thromboembolism, and recurrent endocarditis. HF is treated with vasodilators, positive inotropes, β-adrenergic blockers, diuretics, and a low- sodium diet. The calcium channel blockers diltiazem (Cardizem) and verapamil (Calan, Isoptin) are contraindicated for patients with aortic regurgitation because they decrease ventricular contractility and may cause bradycardia. Anticoagulant therapy prevents and treats systemic or pulmonary emboli. It is used prophylactically in patients with atrial fibrillation. Atrial dysrhythmias are common and treated with calcium channel blockers, β-adrenergic blockers, antidysrhythmia drugs, or electrical cardioversion. Repair, rather than replacement, of a cardiac valve is referred to as valvuloplasty. We see the non-surgical interventions used more on our elderly population who may not do well on bypass. The type of valvuloplasty depends on the cause and type of valve dysfunction. Repair may be made to commissures between the leaflets in a procedure known as commissurotomy, to the annulus of the valve by annuloplasty, to leaflets, or to chordae by chordoplasty. Valvular reconstructions has several advantages: Eliminates need for long-term anticoagulation, Decreases risk of thromboembolism and endocarditis, Decreases need for reoperation, Increases survival rates. Common procedures: Commissurotomy (moderate mitral stenosis), Annuloplasty or prosthetic ring. For high-risk patients and those who are not suitable for open-heart surgery, another option is now available - transcatheter aortic valve replacement (TAVR) - a less invasive procedure that does not require open-heart surgery; it is indicated for relief of aortic stenosis in patients with symptomatic heart disease due to severe native calcific aortic stenosis. There is a good video on TAVR http://newheartvalve.com/hcp/tavr-overview that you may want to view An alternative treatment for some patients with valvular heart disease is the percutaneous transluminal balloon valvuloplasty (PTBV) procedure. During PTBV, the fused commissures are split open. Balloon valvuloplasty is used for mitral, tricuspid, and pulmonic stenosis, and less often for aortic stenosis. The procedure is done in the cardiac catheterization laboratory. It involves threading a balloon- tipped catheter from the femoral artery or vein to the stenotic valve. The balloon is inflated in an attempt to separate the valve leaflets as you see in these figures. A single- or double-balloon technique may be used for the PTBV procedure. Currently, the use of a single Inoue balloon with hourglass shape allows sequential inflation and is the most popular because it is easy and has good results with few complications (e.g., left ventricular perforation). The PTBV procedure is generally indicated for older adults and for those who are poor surgery candidates. Mitral balloon valvuloplasty involves advancing one or two catheters into the right atrium, through the atrial septum into the left atrium, across the mitral valve, and into the left ventricle. The balloon has three sections with progressively greater resistance to inflation. The balloon first expands in the ventricle to help position the catheter at the valve. The second section of the balloon expands above the valve, holding the catheter across the valve. Finally, the middle section of the balloon is inflated for 10 to 15 seconds under fluoroscopic control until the waist of the balloon is no longer visible. All patients have some degree of mitral regurgitation after the procedure. Other possible complications include bleeding from the catheter insertion sites, emboli resulting in complications such as strokes, and, rarely, left-to- right atrial shunts through the atrial septal defect created during the procedure. The long-term results of PTBV are similar to surgical commissurotomy. Mitral commissurotomy (valvulotomy) is the procedure of choice for patients with pure mitral stenosis. Open surgical valvuloplasty involves repair of the valve by suturing the torn leaflets, chordae tendineae, or papillary muscles. It is primarily used to treat mitral or tricuspid regurgitation. Minimally invasive valvuloplasty surgery involves a mini-sternotomy or parasternal approach. It may include robotic and thoracoscopic surgical systems. Results compare with those of the open procedure. In addition, shorter lengths of stay, fewer blood transfusions, less pain, and lower risk of sternal infection and postoperative atrial fibrillation have been reported. Damage to cardiac valve leaflets may result from stretching, shortening, or tearing. Leaflet repair for elongated, ballooning, or other excess tissue leaflets is removal of the extra tissue. Valve leaflet resection and repair with a ring annuloplasty. This figure shows repaire for mitral valve regurgitation: the section indicated by dashed lines is excised; then approximation of edges and suturing showing completed valvuloplasty, leaflet repair, and annuloplasty ring. For patients with mitral or tricuspid regurgitation, further valve repair or reconstruction using annuloplasty is an option. Annuloplasty is repair of the valve annulus (i.e., junction of valve leaflets and muscular heart wall). General anesthesia and cardiopulmonary bypass are required for most annuloplasties. The procedure narrows the diameter of the valve’s orifice and is a useful treatment for valvular regurgitation. The repair prevents progressive regurgitation. Leaflets of the valve are sutured to a ring, creating an annulus of the desired size. When the ring is in place, tension created by moving blood and the contracting heart is borne by the ring rather than by the valve or a suture line. The decision for surgical intervention depends on the patient’s clinical state using the New York Heart Association classification system for functional disability. The type of surgery can be valve repair or valve replacement. The procedure that is used depends on the valves involved, the pathology and severity of the disease, and the patient’s clinical condition. Valve repair is usually the surgical procedure of choice. It has a lower operative mortality rate than valve replacement and is often used in mitral or tricuspid valvular heart disease. Although valve repair avoids the risks of replacement, it may not restore total valve function. A wide variety of prosthetic valves are available for use. Here you see cmechanical and tissue valve replacements. A. Bileaflet (St. Jude, mechanical). B. Caged ball valve (mechanical). C. Tilting-disk valve (mechanical). D. Porcine heterograft valve (biologic). E. Transcatheter aortic valve. F. Transcatheter aortic valve. Desirable valves are non- thrombogenic and durable, and create minimal stenosis. Prosthetic valves are categorized as mechanical or biologic (tissue) valves. Mechanical valves are manufactured from man-made materials and consist of combinations of metal alloys, pyrolite carbon, and Dacron. Biologic valves are constructed from bovine, porcine (Xenografts), and human (cadaver) cardiac tissue and usually contain some man-made materials. Question: Which of the following patients would best benefit from a mechanical valve? B. A 52-year-old with aortic stenosis: A mechanical valve is more durable and people with long life expectancy may benefit from a mechanical valve. They will require anticoagulant therapy. Older patients are at higher risk for the anticoagulant therapy and may benefit with the biological valves. Females of child bearing age may want to have children – cannot be on warfarin. For patients over age 65, durability is less important than the risks of bleeding from anticoagulants, so most receive a biologic valve. Mechanical valves are more durable however they may make audible sounds and the patient needs lifelong anticoagulation. Biologic valves are constructed from bovine, porcine, and human (cadaver) cardiac tissue and usually contain some man-made materials. Biologic valves are not as durable and only require short term (3 months) anticoagulation. Teach importance prophylactic antibiotic regimen before invasive procedures. You Tube has a very good video showing a valve replacement: http://www.youtube.com/watch? v=AXLOo-Eznzc Patients who have undergone percutaneous balloon valvuloplasty with or without percutaneous valve replacement will need to be assessed for signs and symptoms of heart failure and emboli: listening for changes in heart sounds at least every 4 hours, and providing the patient with the same care as for postprocedure cardiac catheterization or percutaneous transluminal coronary angioplasty. After undergoing percutaneous balloon valvuloplasty, the patient usually remains in the hospital for 24 to 48 hours. Patients who have undergone surgical valvuloplasty or valve replacements are admitted to the ICU. Vital signs are assessed every 5 to 15 minutes until stable and then every 2 to 4 hours. IV medications may be needed to increase or decrease blood pressure and treat dysrhythmias or altered heart rates. Patient assessments are conducted hourly initially and then every 2-4 focusing on neurologic, respiratory, and cardiovascular systems. (See Chart 27-13 in Chapter 27, which presents a plan of nursing care for a patient recovering from cardiac surgery.) Patient education is very important so they understand about anticoagulant therapy, explaining the need for frequent follow-up appointments and blood laboratory studies. Patients who take warfarin (Coumadin) have individualized target international normalized ratios, usually between 2 and 3.5 for mitral valve replacement and 1.8 and 2.2 for aortic valve replacement. Patients who have been treated with an annuloplasty ring or a tissue valve replacement usually require anticoagulation for only 3 months unless there are other risk factors such as atrial fibrillation or a history of thromboembolism. Aspirin is prescribed with warfarin for patients with bioprostheses or at high risk for embolic events (e.g., history of embolic event or having two or more preexisting conditions: diabetes, hypertension, coronary artery disease, congestive heart failure, older than 75 years). Patients with a mechanical valve prosthesis (including annuloplasty rings and other prosthetic materials used in valvuloplasty) require education to prevent infective endocarditis. Patients may be at risk for infective endocarditis that results from bacteria entering the bloodstream and adhering to abnormal valve structures or prosthetic devices.. Antibiotic prophylaxis is necessary before dental procedures involving manipulation of gingival tissue, the periapical area of the teeth, or perforation of the oral mucosa (not including routine anesthetic injections, placement of orthodontic brackets, or loss of deciduous teeth). Antibiotic therapy should also be used before invasive procedures especially those involving the respiratory tract (e.g., biopsy of respiratory mucosa, tonsillectomy, and adenectomy). Cardiomyopathy is disease of the heart muscle that is associated with cardiac dysfunction. It is classified according to the structural and functional abnormalities of the heart muscle: dilated cardiomyopathy (DCM), hypertrophic cardiomyopathy (HCM) , restrictive or constrictive cardiomyopathy(RCM), arrhythmogenic right ventricular cardiomyopathy/dysplasia (ARVC/D), and unclassified cardiomyopathy. A patient may have pathology representing more than one of these classifications, such as a patient with HCM developing dilation and symptoms of DCM. Ischemic cardiomyopathy is a term frequently used to describe an enlarged heart caused by coronary artery disease, which is usually accompanied by heart failure. In 2006, the American Heart Association proposed a set of Contemporary Classifications. Under this classification system, cardiomyopathies are divided into two major groups based on predominant organ involvement. These include primary cardiomyopathies (genetic, nongenetic, and acquired), which are focused primarily on the heart muscle, and secondary cardiomyopathies, which show myocardial involvement secondary to the influence of a vast list of disease. We are going to focus on the primary cardiomyopathies. The pathophysiology of all cardiomyopathies is a series of events that culminate in impaired cardiac output. Decreased stroke volume stimulates the sympathetic nervous system and the renin–angiotensin–aldosterone response, resulting in increased systemic vascular resistance and increased sodium and fluid retention, which place an increased workload on the heart. Dilated cardiomyopathy (DCM) is the most common form of cardiomyopathy and is distinguished by significant dilation of the ventricles without simultaneous hypertrophy or systolic dysfunction. The ventricles have elevated systolic and diastolic volumes but a decreased ejection fraction. There are many conditions and diseases may cause DCM, including pregnancy, heavy alcohol intake, viral infection, chemotherapeutic medications, thyrotoxicosis, myxedema, persistent tachycardia, and Chagas disease. When the causative factor cannot be identified, the diagnosis is idiopathic DCM, which accounts for the largest subset with most being linked to familial genetics. Microscopic examination of the muscle tissue shows diminished contractile elements (actin and myosin filaments) of the muscle fibers and diffuse necrosis of myocardial cells. The result is poor systolic function. The structural changes decrease the amount of blood ejected from the ventricle with systole, increasing the amount of blood remaining in the ventricle after contraction. Less blood is then able to enter the ventricle during diastole, increasing end-diastolic pressure and eventually increasing pulmonary and systemic venous pressures. Altered valve function, usually regurgitation, can result from an enlarged stretched ventricle. Poor blood flow through the ventricle may also cause ventricular or atrial thrombi, which may embolize to other locations in the body. Early diagnosis and treatment can prevent or delay significant symptoms and sudden death from DCM. Restrictive cardiomyopathy (RCM) is characterized by diastolic dysfunction caused by rigid ventricular walls that impair diastolic filling and ventricular stretch. Systolic function is usually normal. RCM may be associated with amyloidosis and other such infiltrative diseases, but the cause is idiopathic in most cases. Signs and symptoms are similar to constrictive pericarditis and include dyspnea, nonproductive cough, and chest pain. Echocardiography, as well as measurement of pulmonary artery systolic pressure, pulmonary artery wedge pressure, and central venous pressure is used to differentiate the two conditions. Hypertrophic cardiomyopathy (HCM) is an autosomal dominant condition, occurring in men, women, and children. Genetic testing may help identify those at risk before there are clinical manifestations of the disease. Echocardiograms, 12-lead ECGs, and complete history and physical examinations are typically performed every 12 to 18 months from age 12 to 18 years. These screenings may extend to every 5 years from 18 to 70 years of age in susceptible individuals. Doppler echocardiography has been used for many but the emergence of tissue Doppler imaging (TDI) has been useful in showing dysfunction of the myocardium despite preserved systolic function. Cardiovascular MRI has also become a more prevalent diagnostic tool given its high-resolution tomographic imaging capabilities and is often used in concert with traditional echocardiograms for diagnosis. In HCM, the heart muscle asymmetrically increases in size and mass, especially along the septum. HCM often affects nonadjacent areas of the ventricle. The increased thickness of the heart muscle reduces the size of the ventricular cavities and causes the ventricles to take a longer time to relax after systole. During the first part of diastole, it is more difficult for the ventricles to fill with blood. The atrial contraction at the end of diastole becomes critical for ventricular filling and systolic contraction. Cardiac muscle cells normally lie parallel to and end to end with each other. The hypertrophied cardiac muscle cells are disorganized, oblique, and perpendicular to each other, decreasing the effectiveness of contractions and possibly increasing the risk of dysrhythmias such as ventricular tachycardia and ventricular fibrillation. In HCM, the coronary arteriole walls are thickened, which decrease the internal diameter of the arterioles. The narrow arterioles restrict the blood supply to the myocardium, causing numerous small areas of ischemia and necrosis. The necrotic areas of the myocardium ultimately fibrose and scar, further impeding ventricular contraction. Arrhythmogenic Right Ventricular Cardiomyopathy/Dysplasia (ARVC/D) occurs when the myocardium is progressively infiltrated and replaced by fibrous scar and adipose tissue. Initially, only localized areas of the right ventricle are affected, but as the disease progresses, the entire heart is affected. Eventually, the right ventricle dilates and develops poor contractility, right ventricular wall abnormalities, and dysrhythmias. ARVC/D is genetic and an uncommon form of inherited heart muscle disease and often is not recognized. Palpitations or syncope may develop between 15 and 40 years of age. ARVC/D should be considered in patients with ventricular tachycardia originating in the right ventricle (i.e., a left bundle branch block configuration on ECG) or sudden death, especially among young athletes. First-degree blood relatives should be screened for the disease with a 12-lead ECG, Holter monitor, echocardiography, and a cardiac MRI. Some patients affected by dysrhythmias may benefit from having an ICD placed. Unclassified cardiomyopathies are different from or have characteristics of more than one of the previously described types and are caused by fibroelastosis, noncompacted myocardium, systolic dysfunction with minimal dilation, and mitochondrial diseases. Examples can include left ventricular noncompaction and stress-induced cardiomyopathy. Clinical Manifestations: Patients with cardiomyopathy may remain stable and without symptoms for many years. As the disease progresses, so do the symptoms. Frequently, dilated or restrictive cardiomyopathy is first diagnosed when the patient presents with signs and symptoms of heart failure: PND, cough (especially with exertion), and orthopnea, which may lead to a misdiagnosis of bronchitis or pneumonia. Other symptoms include fluid retention, peripheral edema, and nausea, which are caused by poor perfusion of the gastrointestinal system. The patient also may experience chest pain, palpitations, dizziness, nausea, and syncope with exertion. However, with HCM, cardiac arrest may be the initial manifestation in young people, including athletes. Regardless of type and cause, cardiomyopathy may lead to severe heart failure, lethal dysrhythmias, and death. The mortality rate is highest for African Americans and older adults. Assessment and Diagnostics: Physical examination at early stages may reveal tachycardia and extra heart sounds such as murmurs. With disease progression, examination also reveals signs and symptoms of heart failure. Diagnosis is usually made from findings disclosed by the patient history and by ruling out other causes of heart failure such as myocardial infarction. The echocardiogram is one of the most helpful diagnostic tools because the structure and function of the ventricles can be observed easily. Cardiac MRI may also be used, particularly to assist with the diagnosis of HCM. ECG demonstrates dysrhythmias and changes consistent with left ventricular hypertrophy. In ARVC/D, there often is a small deflection (an epsilon wave) at the end of the QRS. The chest x-ray reveals heart enlargement and possibly pulmonary congestion. Cardiac catheterization is sometimes used to rule out coronary artery disease as a causative factor. Endomyocardial biopsy may be performed to analyze myocardial cells. Medical management is directed toward identifying and managing possible underlying or precipitating causes; correcting the heart failure with medications, a low-sodium diet, and an exercise/rest regimen; and controlling dysrhythmias with antiarrhythmic medications and possibly with an implanted electronic device, such as an ICD. Systemic anticoagulation to prevent thromboembolic events is usually recommended. If the patient has signs and symptoms of congestion, fluid intake may be limited to 2 L each day. Patients with HCM should avoid dehydration and may need beta-blockers to maintain cardiac output and minimize the risk of left ventricular outflow tract (LVOT) obstruction during systole. Patients with HCM or RCM may need to limit physical activity and avoid excessive weight gain to avoid a life-threatening dysrhythmia. To date, amiodarone is the only drug shown to reduce the incidence of arrhythmogenic sudden cardiac death. A pacemaker may be implanted to alter the electrical stimulation of the muscle and prevent the forceful hyperdynamic contractions that occur with HCM. Atrial-ventricular and biventricular pacing have been used to decrease symptoms and obstruction of the LVOT. For some patients with DCM and HCM, biventricular pacing (also known as cardiac resynchronization therapy or CRT) increases the ejection fraction and reverses some of the structural changes in the myocardium. Nonsurgical septal reduction therapy, has been used to treat obstructive HCM. This procedure is oftentimes indicated for patients with advanced age, patients with high operative risk or those with a preference to avoid surgery. The patient may develop a left anterior hemibranch block or left bundle branch block. Nitrates and morphine are not used because coronary artery dilation is contraindicated. Surgical Management: When heart failure progresses and medical treatment is no longer effective, surgical intervention, including heart transplantation, is considered. However, because of the limited number of organ donors, many patients die waiting for transplantation. In some cases, a ventricular assist device (VAD) is implanted to support the failing heart until a suitable donor heart becomes available. When patients with HCM become symptomatic despite medical therapy surgery is considered: usually a myectomy, in which some of the heart tissue is excised. In some of the surgery cases, the surgeon may also need to perform concomitant mitral valve surgery. The primary complication of all procedures is dysrhythmia. Additional complications include postoperative surgical complications such as pain, ineffective airway clearance, deep vein thrombosis, risk of infection, and delayed surgical recovery. Because of advances in surgical techniques and immunosuppressive therapies, heart transplantation is now a therapeutic option for patients with end-stage heart disease. Cyclosporine and tacrolimus are some of the more common immunosuppressants that decrease the body’s rejection of foreign proteins, such as transplanted organs. Unfortunately, these drugs also decrease the body’s ability to resist infections and increase the risk of various cancers, and a satisfactory balance must be achieved between suppressing rejection and avoiding infection. In the United States, approximately 2,400 heart transplants are performed annually, a number that has now plateaued over the previous years. Cardiomyopathy, ischemic heart disease, valvular disease, rejection of previously transplanted hearts, and congenital heart disease are the most common indications for transplantation. Typical candidates have severe symptoms uncontrolled by medical therapy, no other surgical options, and a prognosis of less than 1 to 2 years to live. The person’s age, pulmonary status, other chronic health conditions, psychosocial status, family support, infections, history of other transplantations, adherence to therapeutic regimens, and current health status are considered. When a donor heart becomes available, UNOS generates a list of potential recipients on the basis of ABO blood group compatibility, the body sizes of the donor and the potential recipient, age, severity of illness, length of time on the waiting list, and the geographic locations of the donor and potential recipient. Distance is a factor because postoperative function depends on the heart being implanted within 4 hours of harvest from the donor. Some patients are candidates for more than one organ transplant (e.g., heart–lung, heart–kidney, heart–liver). Orthotopic transplantation is the most common surgical procedure for cardiac transplantation. Some surgeons prefer to remove the recipient’s heart but leave a portion of the recipient’s atria (with the vena cava and pulmonary veins) in place, which is known as the biatrial technique. However, this technique has been modified to a more common approach called the bicaval technique. This technique includes removal of the recipient’s heart, and the implantation of the donor heart with intact atria at the vena cava and pulmonary veins. This newer approach is associated with decreased AV valve regurgitation, dysrhythmias, and conduction abnormalities. Patients who have had heart transplantations are constantly balancing the risk of rejection with the risk of infection and diseases such as cancer. They must adhere to a complex regimen of diet, medications, activity, follow-up laboratory studies, biopsies of the transplanted heart (to diagnose rejection), and clinic visits. There are three classes of medications that are prescribed for a transplant patient to help minimize rejection: corticosteroids, calcineurin inhibitors, and antiproliferative agents. Because the heart is denerved, the patient will not experience chest pain and medications working on the sympathetic and parasympathetic nerves will not be effective on these patients. Though the transplanted heart usually has a faster rate, bradycardia could occur – atropine will not be effective so this patient would need a pacemaker. The use of cardiopulmonary bypass in cardiovascular surgery and the possibility of performing heart transplantation in patients with end-stage cardiac disease, as well as the desire for a treatment option for patients with such disease who are not transplant candidates, have increased the need for mechanical assist devices. Patients who cannot be weaned from cardiopulmonary bypass and patients in cardiogenic shock may benefit from a period of mechanical heart assistance. The most commonly used device is the intra-aortic balloon pump. This pump decreases the work of the heart during contraction but does not perform the actual work of the heart. The intraaortic balloon pump (IABP) is a device that increases coronary blood flow to the heart muscle and decreases the heart’s workload through a process called counterpulsation. This link explains the process (http://www.youtube.com/watch? v=o11fhdVOYWA) The IABP is placed in the aorta. The IABP and impella are useful in hemodynamically unstable patients because they decrease SVR, PAWP, and PAP leading to improved CO. Several mechanical options are available to sustain HF patients with deteriorating conditions, especially those awaiting cardiac transplantation. Ventricular Assist Devices More complex devices that perform some or all of the pumping function for the heart are being used. These more sophisticated ventricular assist devices (VADs) can circulate as much blood per minute as the heart, if not more (see Fig. 28-10). There are short- and long-term devices available, depending on the indication. Each VAD is used to support one ventricle, although in some instances, two VAD pumps may be used for biventricular support. Additionally, some VADs can be combined with an oxygenator; the combination is called extracorporeal membrane oxygenation (ECMO). The oxygenator–VAD combination is used for the patient whose heart cannot pump adequate blood through the lungs or the body. VADs may be used as (1) a “bridge to recovery” for patients who require temporary assistance for reversible ventricular failure, (2) a “bridge to transplant” for patients with end-stage heart failure until a donor organ becomes available for transplant (most common), and (3) “destination therapy” for patients with end-stage heart failure who are not candidates for or decline heart transplantation and have the VAD implanted for permanent use. As patients spend an increased length of time on the transplant list, and as more VADs are becoming approved for destination therapy, VAD patients are being discharged from the hospital with the devices in place. Complications of VADs include bleeding disorders, hemorrhage, thromboemboli, hemolysis, infection, kidney injury, right-sided heart failure, multisystem failure, and mechanical failure. Nursing care of patients with these mechanical assist devices focuses on assessment for and minimization of these complications as well as providing emotional support and education about the device and the underlying cardiac disease. Management of patients with cardiomyopathies include improvement or maintenance of cardiac output, increased activity tolerance, reduction of anxiety, adherence to the self-care program, increased sense of power with decision-making, and absence of complications. During a symptomatic episode, rest is indicated. Many patients with DCM find that sitting up with their legs down is more comfortable than lying down in a bed. This position is helpful in pooling venous blood in the periphery and reducing preload. Assessing the patient’s oxygen saturation at rest and during activity may assist with determining a need for supplemental oxygen. Oxygen usually is given through a nasal cannula when indicated. Ensuring that medications are taken as prescribed is important to preserving adequate cardiac output. The nurse may assist the patient with planning a schedule for taking medications and identifying methods to remember to follow it, such as associating the time to take a medication with an activity (e.g., eating a meal, brushing teeth). It is important to ensure that patients with DCM avoid verapamil, that patients with HCM avoid diuretics, and that patients with RCM avoid nifedipine to maintain contractility. In patients with HCM, the inotropic action of digoxin may create or worsen LVOT obstruction. Patients with RCM have increased sensitivity to digoxin, and the nurse must anticipate that low doses will be prescribed and assess for digoxin toxicity. It is also important to ensure that the patient receives or chooses food selections that are appropriate for a low- sodium diet. Patients should weight every day and identify any significant change. Another indication of the effect of treatment involves assessment of shortness of breath after activity and comparison to before treatment, as well as a change in the number of pillows needed to comfortably sleep. Patients with low cardiac output may need assistance keeping warm and frequently changing position to stimulate circulation and reduce the possibility of skin breakdown. Patients with HCM must be taught to avoid dehydration. The nurse plans the patient’s activities so that they occur in cycles, alternating rest with activity periods. This benefits the patient’s physiologic status, and it helps educate the patient about the need for planned cycles of rest and activity. For example, after taking a bath or shower, the patient should plan to sit and read a newspaper or engage in other relaxing activities. Suggesting that the patient sit while chopping vegetables, drying their hair, or shaving helps the patient learn to balance rest with activity. The nurse also makes sure that the patient recognizes the symptoms indicating the need for rest and actions to take when the symptoms occur. Patients with HCM or RCM must avoid strenuous activity, isometric exercises, and competitive sports. Spiritual, psychological, and emotional support may be indicated for patients, families, and significant others. Interventions are directed toward eradicating or alleviating perceived stressors. Patients receive appropriate information about cardiomyopathy and self-management activities. It is important to provide an atmosphere in which patients feel free to verbalize concerns and receive assurance that their concerns are legitimate. If the patient is awaiting transplantation or facing death, it is necessary to allow time to discuss these issues. Providing the patient with realistic hope helps reduce anxiety while he or she awaits a donor heart. The nurse helps the patient, family, and significant others with anticipatory grieving. The patient is assisted in identifying the things in life that he or she has lost and their emotional responses to the loss. The nurse assists the patient in identifying the amount of control that he or she still has over life, such as making food choices, managing medications, and working with the patient’s primary provider to achieve the best possible outcomes. A diary in which the patient records food selections and weight may help with understanding the relationship between sodium intake and weight gain and give patients a sense of control over their disease. Some patients can manage a self-titrating diuretic regimen in which they adjust the dose of diuretic to their symptoms. Any of the heart’s three layers may be affected by an infectious process. Infections are named for the layer of the heart most involved in the infectious process: infective endocarditis (endocardium), myocarditis (myocardium), and pericarditis (pericardium). Diagnosis is made primarily on the basis of the patient’s symptoms and echocardiography. Ideal management for all infectious diseases is prevention. IV antibiotics usually are necessary once an infection has developed in the heart. Acute rheumatic fever, which occurs most often in school-age children, may develop after an episode of group A beta-hemolytic streptococcal pharyngitis. Chart 28-3 lists the S&S of Rheumatic Fever. Patients with rheumatic fever may develop rheumatic heart disease as evidenced by a new heart murmur, cardiomegaly, pericarditis, and heart failure. Prompt and effective treatment of “strep” throat with antibiotics can prevent development of rheumatic fever. Streptococcus is spread by direct contact with oral or respiratory secretions. Infective endocarditis is a microbial infection of the endothelial surface of the heart. It usually develops in people with prosthetic heart valves, cardiac devices, or structural cardiac defects. Chart 28-4 lists the risk factors associated with infective endocarditis. It is more common in older adults due to degenerative or calcific valve lesions, reduced immunologic response to infection, and metabolic alterations associated with aging. Staphylococcal endocarditis infections of valves in the right side of the heart are common among IV drug abusers. Hospital-acquired infective endocarditis occurs most often in patients with debilitating disease or indwelling catheters and in patients who are receiving hemodialysis or prolonged IV fluid or antibiotic therapy. Patients taking immunosuppressive medications or corticosteroids are more susceptible to fungal endocarditis. A deformity or injury of the endocardium leads to accumulation of fibrin and platelets (clot formation) on the endocardium. Infectious organisms, usually staphylococci or streptococci, invade the clot and endocardial lesion. Other causative microorganisms include fungi and Rickettsiae. Infection most frequently results in platelets, fibrin, blood cells, and microorganisms that cluster as vegetations on the endocardium. Vegetations may embolize to other tissues throughout the body. As the clot on the endocardium continues to expand, the infecting organism is covered by new clot and concealed from the body’s normal defenses. Infection may erode through the endocardium into underlying structures (e.g., valve leaflets), causing tears or other deformities of valve leaflets, dehiscence of prosthetic valves, deformity of chordae tendineae, or mural abscesses. Onset of infective endocarditis usually is insidious. Signs and symptoms develop from toxic effects of the infection, destruction of heart valves, and embolization of fragments of vegetative growths on the endocardium. Systemic emboli are more common with left-sided heart infective endocarditis when vegetations are large; pulmonary emboli may occur with right-sided heart infective endocarditi Primary presenting symptoms of infective endocarditis are fever and a heart murmur. Fever may be intermittent or absent, especially in patients who are receiving antibiotics or corticosteroids, in older adults, and in those who have heart failure or kidney injury. A heart murmur may be absent initially but develops in almost all patients. Murmurs that worsen over time indicate progressive damage from vegetations or perforation of a valve or rupture of chordae tendineae. n addition to fever and heart murmur, clusters of petechiae may be found on the body. Small, painful nodules (Osler nodes) may be present in pads of fingers or toes. Irregular, red or purple, painless flat macules (Janeway lesions) may be present on palms, fingers, hands, soles, and toes. Hemorrhages with pale centers (Roth spots) caused by emboli may be observed in fundi of the eyes. Splinter hemorrhages (i.e., reddish-brown lines and streaks) may be seen under the proximal half of fingernails and toenails. Petechiae may appear in conjunctiva and mucous membranes. Cardiomegaly, heart failure, tachycardia, or splenomegaly may occur. Central nervous system manifestations of infective endocarditis include headache; temporary or transient cerebral ischemia; and strokes, which may be caused by emboli to cerebral arteries. Embolization may be a presenting symptom; it may occur at any time and may involve other organ systems. Embolic phenomena may occur. Heart failure, which may result from perforation of a valve leaflet, rupture of chordae, blood flow obstruction due to vegetations, or intracardiac shunts from dehiscence of prosthetic valves, indicates a poor prognosis with medical therapy alone and a higher surgical risk. Valvular stenosis or regurgitation, myocardial damage, and mycotic (fungal) aneurysms are potential cardiac complications. First-, second-, and third-degree atrioventricular blocks may occur and are often a sign of a valve ring abscess. Emboli, immunologic responses, abscess of the spleen, mycotic aneurysms, cerebritis, and hemodynamic deterioration may cause complications in other organs. Vague complaints of malaise, anorexia, weight loss, cough, and back and joint pain may be mistaken for influenza. Virulence of the causative organism usually correlates with the speed and degree of symptom development. A definitive diagnosis is made when a microorganism is found in two separate blood cultures, or in a vegetation or abscess. At least 2 sets of blood cultures (each consisting of one aerobic and one anaerobic culture) drawn from different venipuncture sites over a 24-hour period (each set at least 12 hours apart), or every 30 minutes if the patient’s condition is unstable, should be obtained before administration of any antimicrobial agents. Negative blood cultures do not definitely rule out infective endocarditis. Patients may have elevated white blood cell (WBC) counts. In addition, patients may be anemic, have a positive rheumatoid factor, and an elevated erythrocyte sedimentation rate (ESR) or C-reactive protein. A key strategy is primary prevention in high-risk patients. The objective of treatment is to eradicate invading organisms through adequate doses of an appropriate antimicrobial agent. Antibiotic therapy usually is given for 2 to 6 weeks every 4 hours or continuously by IV infusion. Parenteral therapy is given in doses that produce a high serum concentration for a significant period to ensure eradication of the dormant bacteria within dense vegetations. This therapy is often delivered in the patient’s home and is monitored by a home care nurse. Serum levels of the antibiotic and blood cultures are monitored to gauge effectiveness of therapy. If there is insufficient bactericidal activity, increased dosages of the antibiotic are prescribed or a different antibiotic is used. Numerous antimicrobial regimens are in use, but penicillin usually is the medication of choice. In fungal endocarditis, an antifungal agent such as amphotericin B is the usual treatment. Surgical intervention may be required if the infection does not respond to medications or the patient has prosthetic heart valve endocarditis, has a mobile vegetation, has heart failure, has heart block, or develops complications such as a septal perforation. Surgical interventions include valve débridement or excision, débridement of vegetations, débridement and closure of an abscess, and closure of a fistula. Aortic or mitral valve débridement, excision, or replacement. The nurse monitors the patient’s temperature; the patient may have a fever for weeks. The nurse administers antibiotic, antifungal, or antiviral medication as prescribed or educates the patient to take them as prescribed. Patients need enough fluids to keep their urine light yellow. Fever often causes fatigue; rest periods should be planned and activities spaced to give to rest between activities. Good infection control and prevention practices include appropriate hand hygiene by both patients and caregivers. Nonsteroidal anti-inflammatory drugs (NSAIDs) may be prescribed as antipyretics or to decrease the discomfort of fever. Patients may be more comfortable with a light layer of linens and exposure of their skin to air. They may be cooled with a fan, tepid water baths, or cloth compresses; if shivering or piloerection occurs, these interventions should be discontinued due to increased oxygen consumption and potential to further increase of body temperature. Heart sounds are assessed. A new or worsening murmur may indicate dehiscence of a prosthetic valve, rupture of an abscess, or injury to valve leaflets or chordae tendineae. The nurse monitors for signs and symptoms of systemic embolization, or, for patients with right-sided heart endocarditis, for signs and symptoms of pulmonary infarction and infiltrates. In addition, the nurse assesses signs and symptoms of organ damage such as stroke, meningitis, heart failure, myocardial infarction, glomerulonephritis, and splenomegaly. Patient care is directed toward management of infection. Long-term IV antimicrobial therapy often is necessary; therefore, many patients have peripherally inserted central catheters or other long-term IV access. All invasive lines and wounds must be assessed daily for redness, tenderness, warmth, swelling, drainage, or other signs of infection. The patient and family are instructed about activity restrictions, medications, and signs and symptoms of infection. Patients with infective endocarditis are at high risk for another episode of infectious endocarditis. Myocarditis, an inflammatory process involving the myocardium, can cause heart dilation, thrombi on the heart wall (mural thrombi), infiltration of circulating blood cells around the coronary vessels and between the muscle fibers, and degeneration of the muscle fibers themselves. Mortality varies with the severity of symptoms. Most patients with mild symptoms recover completely; however, some patients develop cardiomyopathy and heart failure. Myocarditis usually results from viral, bacterial, rickettsial, fungal, parasitic, metazoal, protozoal, or spirochetal infection. It also may be immune related, occurring after acute systemic infections such as rheumatic fever. It may develop in patients receiving immunosuppressive therapy or in those with infective endocarditis, Crohn’s disease, or systemic lupus. Myocarditis may result from an inflammatory reaction to toxins such as pharmacologic agents used in the treatment of other diseases, ethanol, or radiation. It may begin in one small area of the myocardium and then spread throughout the myocardium. The degree of myocardial inflammation and necrosis determines the degree of interstitial collagen and elastin destruction. The greater the destruction, the greater is the hemodynamic effect and resulting signs and symptoms. It is thought that DCM and HCM are latent manifestations of myocarditis The symptoms of acute myocarditis depend on the type of infection, the degree of myocardial damage, and the capacity of the myocardium to recover. Patients may be asymptomatic, with an infection that resolves on its own. However, they may develop mild-to-moderate symptoms and seek medical attention, often reporting fatigue and dyspnea, syncope, palpitations, and occasional discomfort in the chest and upper abdomen. The most common symptoms are flulike. Patients may also sustain sudden cardiac death or quickly develop severe congestive heart failure. A cardiac MRI is being used more often as a diagnostic tool because of its noninvasive. With contrast, cardiac MRI may be diagnostic and can guide clinicians to sites for endocardial biopsies, which may be diagnostic for an organism or its genome, an immune process, or a radiation reaction causing the myocarditis. Patients without any abnormal heart structure (at least initially) may suddenly develop dysrhythmias or ST–T-wave changes. If the patient has structural heart abnormalities (e.g., systolic dysfunction), a clinical assessment may disclose cardiac enlargement, faint heart sounds (especially S1), pericardial friction rub, a gallop rhythm, or a systolic murmur. The WBC count, C-reactive protein, leukocyte count, and ESR may be elevated Prevention of infectious diseases by means of appropriate immunizations (e.g., influenza, hepatitis) and early treatment appears to be important in decreasing the incidence of myocarditis. Patients are given specific treatment for the underlying cause if it is known and are placed on bed rest to decrease cardiac workload. Bed rest also helps decrease myocardial damage and the complications of myocarditis. In young patients with myocarditis, activities, especially athletics, should be limited for a 6-month period or at least until heart size and function have returned to normal. Physical activity is increased slowly, and the patient is instructed to report any symptoms that occur with increasing activity, such as a rapidly beating heart. If heart failure or dysrhythmia develops, management is essentially the same as for all causes of heart failure and dysrhythmias. Although they are known for their anti-inflammatory effects, NSAIDs should not be used for pain control; they have been shown to be ineffective in relieving the inflammatory process in myocarditis and have been linked to worsening inflammation of the myocardium. This also can contribute to an increased mortality from increased virulence of the pathogen The nurse assesses for resolution of tachycardia, fever, and any other clinical manifestations. The cardiovascular assessment focuses on signs and symptoms of heart failure and dysrhythmias. Patients with dysrhythmias should have continuous cardiac monitoring with personnel and equipment readily available to treat life- threatening dysrhythmias. Patients with myocarditis are sensitive to digitalis. Nurses must closely monitor these patients for digitalis toxicity, which is evidenced by a new onset of dysrhythmia, anorexia, nausea, vomiting, headache, and malaise. The primary provider should be notified immediately if this is suspected Pericarditis refers to an inflammation of the pericardium, which is the membranous sac enveloping the heart. It may be a primary illness, or it may develop during various medical and surgical disorders. For example, pericarditis may occur after pericardiectomy (opening of the pericardium) following cardiac surgery. Pericarditis also may occur 10 days to 2 months after acute myocardial infarction (Dressler syndrome) (Curry, 2014; Mann et al., 2015). Pericarditis may be acute, chronic, or recurring. It is classified either as adhesive (constrictive), because the layers of the pericardium become attached to each other and restrict ventricular filling, or by what accumulates in the pericardial sac: serous (serum), purulent (pus), calcific (calcium deposits), fibrinous (clotting proteins), sanguinous (blood), or malignant (cancer). Pericarditis also may be described as exudative or noneffusive. Causes underlying or associated with pericarditis are listed in Chart 28-5. The inflammatory process of pericarditis may lead to an accumulation of fluid in the pericardial sac (pericardial effusion) and increased pressure on the heart, leading to cardiac tamponade. Frequent or prolonged episodes of pericarditis also may lead to thickening and decreased elasticity of the pericardium, or scarring may fuse the visceral and parietal pericardium. These conditions restrict the heart’s ability to fill with blood (constrictive pericarditis). The pericardium may become calcified, further restricting ventricular expansion during ventricular filling (diastole). With less filling, the ventricles pump less blood, leading to decreased cardiac output and signs and symptoms of heart failure. Restricted diastolic filling may result in increased systemic venous pressure, causing peripheral edema and hepatic failure. Pericarditis may be asymptomatic. The most characteristic symptom of pericarditis is chest pain, although pain also may be located beneath the clavicle, in the neck, or in the left trapezius (scapula) region. Pain or discomfort usually remains fairly constant, but it may worsen with deep inspiration and when lying down or turning. The most characteristic clinical manifestation of pericarditis is a creaky or scratchy friction rub heard most clearly at the left lower sternal border. Other signs may include a mild fever, increased WBC count, anemia, and an elevated ESR or C-reactive protein level. Patients may have a nonproductive cough or hiccup. Dyspnea, as well as respiratory splinting because of pain upon inspiration, and other signs and symptoms of heart failure may occur as a result of pericardial compression due to constrictive pericarditis or cardiac tamponade. The heart rate may increase to maintain cardiac output. The diagnosis most often is made on the basis of history, signs, and symptoms. An echocardiogram may detect inflammation, pericardial effusion or tamponade, and heart failure. It may help confirm the diagnosis and may be used to guide pericardiocentesis (needle or catheter drainage of the pericardium). TEE may be useful in diagnosis but may underestimate the extent of pericardial effusions. CT imaging may be the best diagnostic tool for determining size, shape, and location of pericardial effusions and may be used to guide pericardiocentesis. Cardiac MRI may assist with detection of inflammation and adhesions. Occasionally, a video-assisted pericardioscope-guided biopsy of the pericardium or epicardium is performed to obtain tissue samples for culture and microscopic examination. Because the pericardial sac surrounds the heart, a 12-lead ECG may show concave ST elevations in many, if not all, leads (with no reciprocal changes) and may show depressed PR segments or atrial dysrhythmias Objectives of pericarditis management are to determine the cause, administer therapy for treatment and symptom relief, and detect signs and symptoms of cardiac tamponade. When cardiac output is impaired, the patient is placed on bed rest until fever, chest pain, and friction rub have subsided. Analgesic medications and NSAIDs such as aspirin or ibuprofen (Motrin) may be prescribed for pain relief during the acute phase. These agents also hasten reabsorption of fluid in patients with rheumatic pericarditis. Indomethacin (Indocin) is contraindicated because it may decrease coronary blood flow. Colchicine or corticosteroids may be prescribed if the pericarditis is severe or if the patient does not respond to NSAIDs. Colchicine also may be used instead of NSAIDs during the acute phase. Pericardiocentesis, a procedure in which some pericardial fluid is removed, rarely is necessary. It may be performed to assist in identification of the cause or relieve symptoms, especially if there are signs and symptoms of heart failure or tamponade. Pericardial fluid is cultured if bacterial, tubercular, or fungal disease is suspected; a sample is sent for cytology if neoplastic disease is suspected. A pericardial window, a small opening made in the pericardium, may be performed to allow continuous drainage into the chest cavity. Surgical removal of tough encasing pericardium (pericardiectomy) may be necessary to release both ventricles from constrictive and restrictive inflammation and scarring. Patients with acute pericarditis require pain management with analgesics, assistance with positioning, and psychological support. Patients with chest pain often benefit from education and reassurance that the pain is not due to a heart attack. Pain may be relieved with a forward-leaning or sitting position. To minimize complications, the nurse helps the patient with activity restrictions until pain and fever subside. As the patient’s condition improves, the nurse encourages gradual increases of activity. However, if pain, fever, or friction rub recurs, activity restrictions must be resumed. The nurse educates the patient and family about a healthy lifestyle to enhance the patient’s immune system. Nurses caring for patients with pericarditis must be alert to cardiac tamponade. The nurse monitors the patient for heart failure. Patients with hemodynamic instability or pulmonary congestion are treated as if they had heart failure The primary symptom of pericarditis is pain, which is assessed by evaluating the patient in various positions. The nurse tries to identify whether pain is influenced by respiratory movements, while holding an inhaled breath or holding an exhaled breath; by flexion, extension, or rotation of the spine, including the neck; by movements of shoulders and arms; by coughing; or by swallowing. Recognizing events that precipitate or intensify pain may help establish a diagnosis and differentiate pain of pericarditis from pain of myocardial infarction. When pericardial surfaces lose their lubricating fluid because of inflammation, a pericardial friction rub occurs. The rub is audible on auscultation and is synchronous with the heartbeat. However, it may be elusive and difficult to detect. A pericardial friction rub is diagnostic of pericarditis. It is a creaky or scratchy sound and is louder at the end of exhalation. Nurses should monitor for pericardial friction rub by placing the diaphragm of the stethoscope tightly against the patient’s thorax and auscultating the left sternal edge in the fourth intercostal space, which is the site where the pericardium comes into contact with the left chest wall. The rub may be heard best when a patient is sitting and leaning forward. If there is difficulty in distinguishing a pericardial friction rub from a pleural friction rub, the patient is asked to hold their breath; a pericardial friction rub will continue to be heard. The patient’s temperature is monitored frequently. Pericarditis may cause an abrupt onset of fever in a patient who has been afebrile. Relief of pain is achieved by rest. Because sitting upright and leaning forward is the posture that tends to relieve pain, chair rest may be more comfortable. The nurse instructs the patient to restrict activity until pain subsides. As chest pain and friction rub abate, activities of daily living may be resumed gradually. If the patient is taking analgesics, antibiotics, or corticosteroids for pericarditis, their responses are monitored and recorded. Patients taking NSAIDs or colchicine are assessed for gastrointestinal adverse effects. If chest pain and friction rub recur, bed rest or chair rest is resumed. Abnormal accumulation of fluid between the pericardial linings is called pericardial effusion. Most patients have no effects or symptoms. However, enough fluid can accumulate to constrict the myocardium, impairing ventricular filling and the myocardium’s ability to pump, a condition known as cardiac tamponade. Failure to identify and treat this problem can lead to death. Signs and symptoms of cardiac tamponade may begin with the patient reporting shortness of breath, chest tightness, or dizziness. The nurse may observe that the patient is becoming progressively more restless. Assessment of blood pressure may reveal a decrease of 10 mm Hg or more in systolic blood pressure during inspiration (pulsus paradoxus). Usually, the systolic pressure decreases and the diastolic pressure remains stable; hence, the pulse pressure narrows. The patient usually has tachycardia, and ECG voltage may be decreased or QRS complexes may alternate in height (electrical alternans). Heart sounds may progress from distant to imperceptible. Blood continues to return to the heart from the periphery but cannot flow into the heart to be pumped back into the circulation. The patient develops jugular vein distention and other signs of rising central venous pressure. Beck triad (hypotension, muffled heart sounds, and an elevated jugular venous pressure) is a useful diagnostic parameter of severe tamponade. In such situations, the nurse notifies the primary provider immediately and prepares to assist with diagnostic echocardiography and pericardiocentesis. The nurse stays with the patient and continues to assess and record signs and symptoms while intervening to decrease patient anxiety. Question: A patient with restrictive cardiomyopathy taking digoxin presents with symptoms of anorexia, nausea, vomiting, headache, and malaise. What should the nurse expect to be included in the plan of care for this patient? B. The patient’s digoxin dose will be decreased. Rationale: Patients with restrictive cardiomyopathy are sensitive to digitalis. Nurses must closely monitor these patients for digitalis toxicity, which is evidenced by dysrhythmia, anorexia, nausea, vomiting, headache, and malaise. This patient presents with symptoms of digoxin toxicity, so a decrease in dosage should be anticipated. These patients should avoid nifedipine, and they do not need to be admitted to the ICU [Show More]
Last updated: 1 year ago
Preview 1 out of 18 pages
Instant download
Buy this document to get the full access instantly
Instant Download Access after purchase
Add to cartInstant download
Reviews( 0 )
Document information
Connected school, study & course
About the document
Uploaded On
Oct 21, 2021
Number of pages
18
Written in
Additional information
This document has been written for:
Uploaded
Oct 21, 2021
Downloads
0
Views
64


.png)