Chemistry > AQA Question Papers > AQA_AS Level Chemistry Paper 1_Question Paper 2022 | Practice Paper 1 (All)
AQA_AS Level Chemistry Paper 1_Question Paper 2022 | Practice Paper 1
Document Content and Description Below
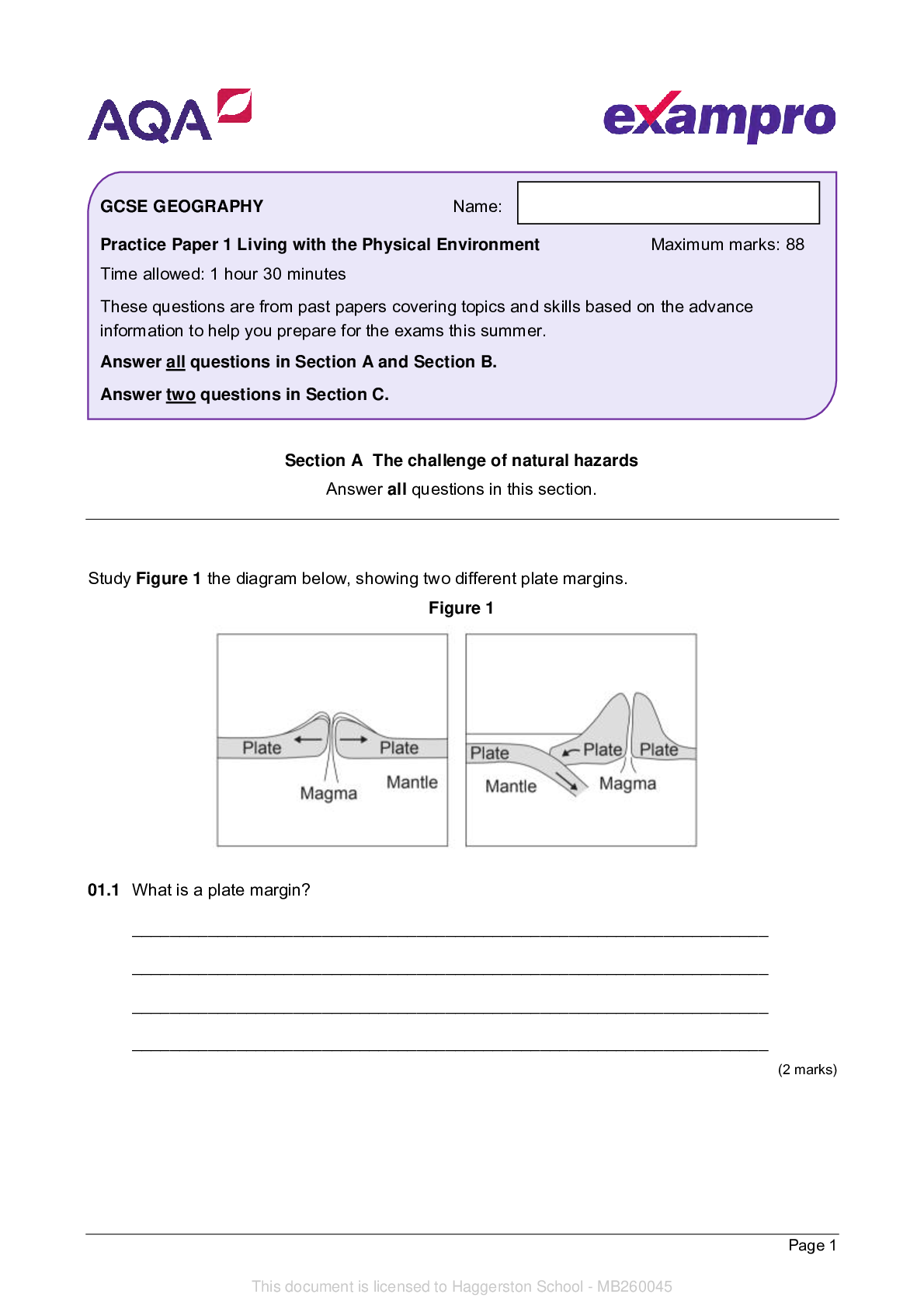
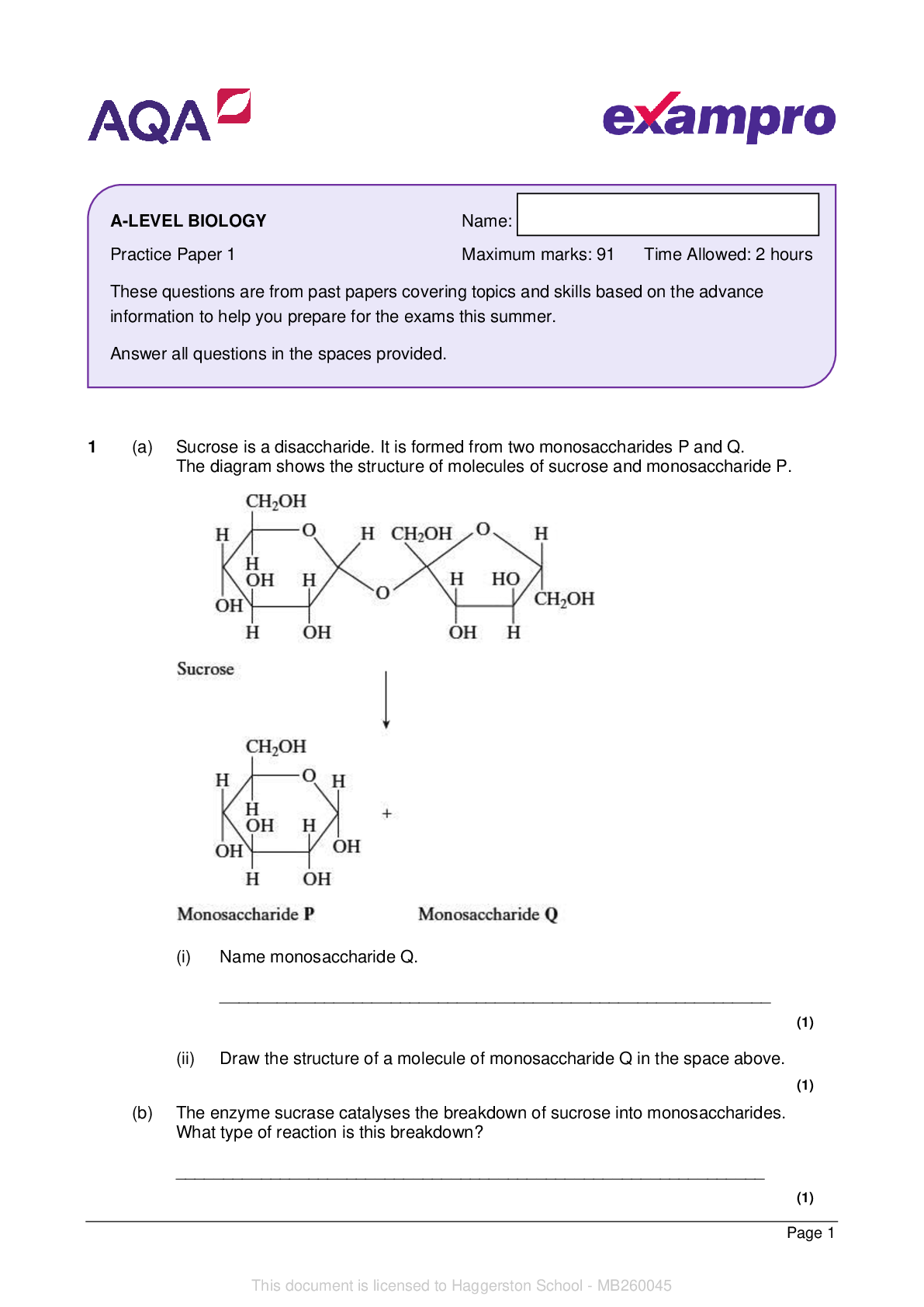
AQA_AS Level Chemistry Paper 1_Question Paper 2022 | Practice Paper 1 - - - - 1 In this question, give all values of pH to 2 decimal places. (a) The ionic product of water has the symbol Kw (i)... Write an expression for the ionic product of water. __________________________________________________________ (1) (ii) At 42°C, the value of Kw is 3.46 × 10−14 mol2 dm−6. Calculate the pH of pure water at this temperature (to 2 decimal places). __________________________________________________________ __________________________________________________________ __________________________________________________________ __________________________________________________________ (2) (iii) At 75 °C, a 0.0470 mol dm–3 solution of sodium hydroxide has a pH of 11.36. Calculate a value for Kw at this temperature. __________________________________________________________ __________________________________________________________ __________________________________________________________ __________________________________________________________ (2) Name: Practice Paper 1 Maximum marks: 105 Time allowed: 2 hours These questions are from past papers covering topics and skills based on the advance information to help you prepare for the exams this summer. Answer all questions in the spaces provided. You will need the Periodic Table/Data Booklet This document is licensed to Haggerston School - MB260045 AS-LEVEL CHEMISTRYPage 2 (b) Methanoic acid (HCOOH) slightly dissociates in aqueous solution. (i) Write an equation for this dissociation. __________________________________________________________ (1) (ii) Write an expression for the acid dissociation constant Ka for methanoic acid. __________________________________________________________ __________________________________________________________ (1) (iii) The value of Ka for methanoic acid is 1.78 × 10−4 mol dm−3 at 25 °C. Calculate the pH of a 0.0560 mol dm−3 solution of methanoic acid. __________________________________________________________ __________________________________________________________ __________________________________________________________ __________________________________________________________ __________________________________________________________ __________________________________________________________ (3) (iv) The dissociation of methanoic acid in aqueous solution is endothermic. Deduce whether the pH of a solution of methanoic acid will increase, decrease or stay the same if the solution is heated. Explain your answer. - - - [Show More]
Last updated: 1 year ago
Preview 1 out of 17 pages

Also available in bundle (2)

AQA_AS Level Chemistry Paper 1_Question Paper & Mark Scheme_2022 | Practice Paper 1
AQA_AS Level Chemistry Paper 1_Question Paper 2022 | Practice Paper 1 AQA_AS Level Chemistry Paper 1_Mark Scheme 2022 | Practice Paper 1
By A-Level Guru 1 year ago
$11.5
2

AQA_AS Level Chemistry Paper 1 & 2_EXAMPRO | Question Paper & Mark Scheme_2022
AQA_AS Level Chemistry Paper 1_Question Paper 2022 | Practice Paper 1 AQA_AS Level Chemistry Paper 1_Mark Scheme 2022 | Practice Paper 1 AQA_AS Level Chemistry Paper 2_Question Paper 2022 | Practice...
By A-Level Guru 1 year ago
$19.5
4
Reviews( 0 )
Document information
Connected school, study & course
About the document
Uploaded On
Jun 02, 2022
Number of pages
17
Written in
Additional information
This document has been written for:
Uploaded
Jun 02, 2022
Downloads
0
Views
648