CHEM 103 MODULE 3 EXAM 2022
Document Content and Description Below
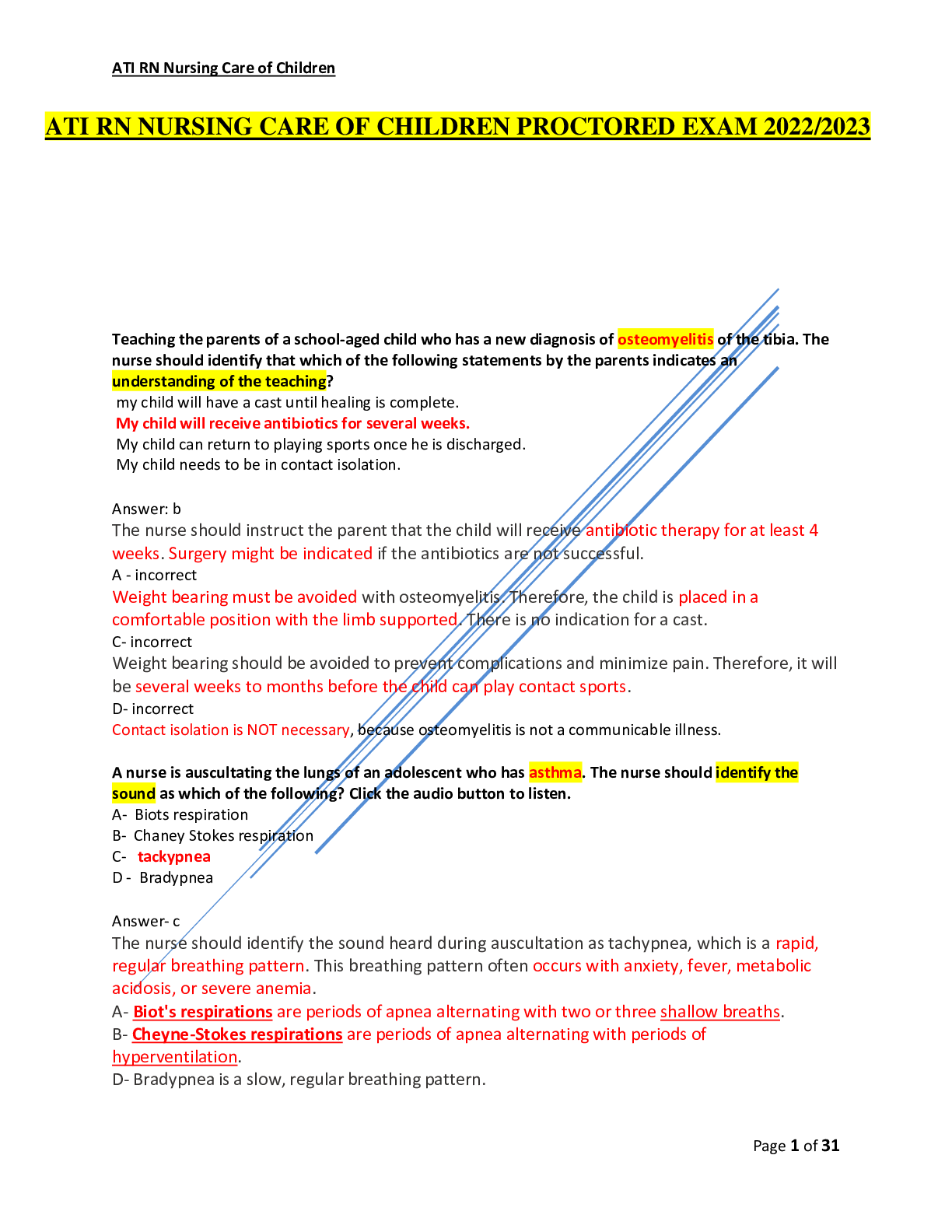
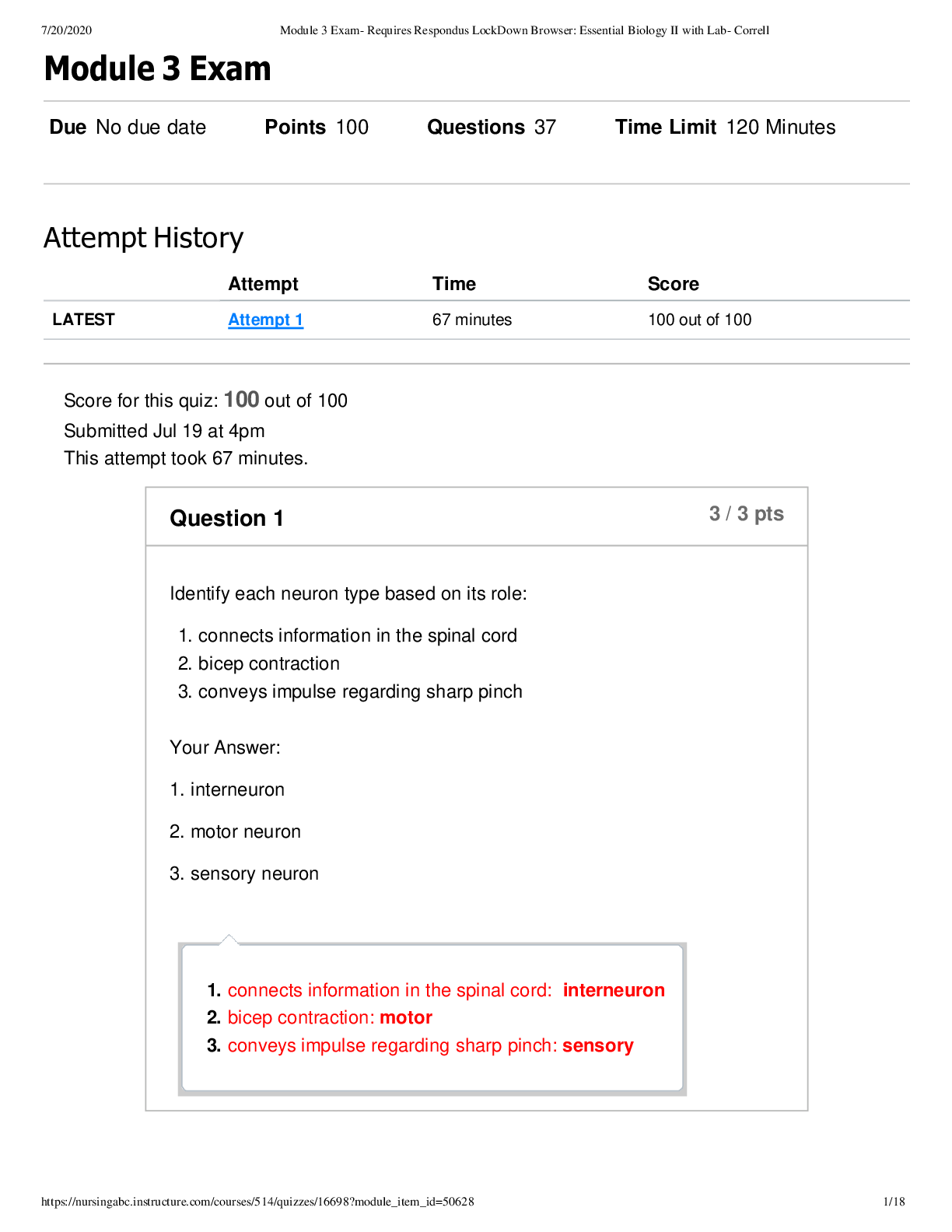
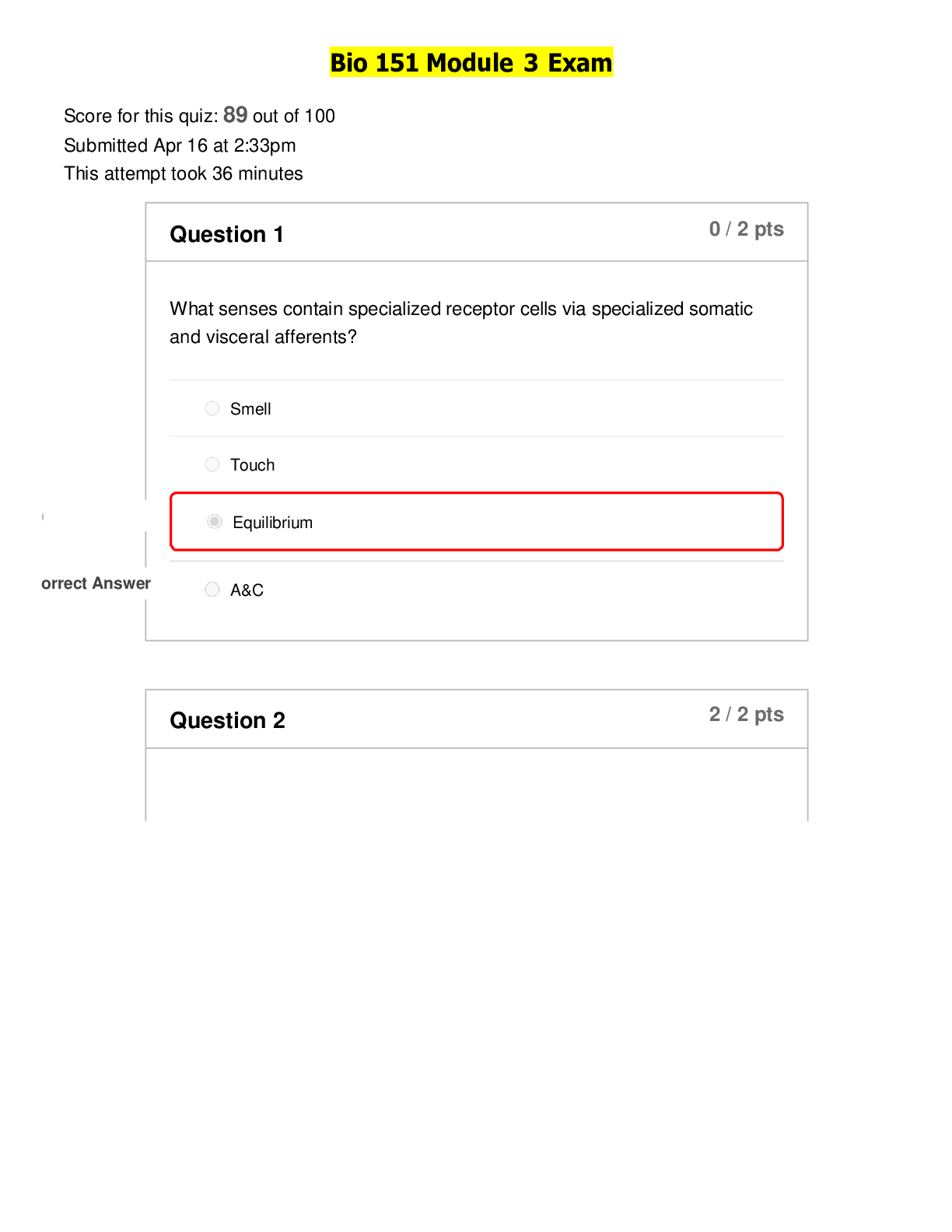
CHEM 103 MODULE 3 EXAM 2022 Click this link to access the Periodic Table. This may be helpful throughout the exam. A reaction between HCl and NaOH is being studied in a styrofoam co ffee cup with NO ... lid and the heat given off is measured by means of a thermometer immersed in the reaction mixture. Enter the correct thermochemistry term to describe the item listed. 1. The type of thermochemical process 2. The amount of heat released in the reaction of HCl with NaOH 1. Heat given off = Exothermic process 2. The amount of heat released = Heat of reaction Question 2 Click this link to access the Periodic Table. This may be helpful throughout the exam. 1. Show the calculation of the final temperature of the mixture when a 22.8 gram sample of water at 74.6oC is added to a 14.3 gram sample of water at 24.3oC in a coffee cup calorimeter. c (water) = 4.184 J/g oC 2. Show the calculation of the energy involved in freezing 54.3 grams of water at 0oC if the Heat of Fusion for water is 0.334 kJ/g 1. - (mwarn H2O x cwarn H2O x ∆twarn H2O) = (mcool H2O x ccool H2O x ∆tcool H2O) [Show More]
Last updated: 1 year ago
Preview 1 out of 8 pages

Buy this document to get the full access instantly
Instant Download Access after purchase
Add to cartInstant download
We Accept:

Reviews( 0 )
$9.00
Document information
Connected school, study & course
About the document
Uploaded On
Sep 14, 2022
Number of pages
8
Written in
Additional information
This document has been written for:
Uploaded
Sep 14, 2022
Downloads
0
Views
34






.png)







 (1).png)


