*NURSING > CASE STUDY > NR-507 Week 7 Assignment: CNS: Sensory and Motor Disorders (E-dapt Content) | aLREADY GRADED A (All)
NR-507 Week 7 Assignment: CNS: Sensory and Motor Disorders (E-dapt Content) | aLREADY GRADED A
Document Content and Description Below
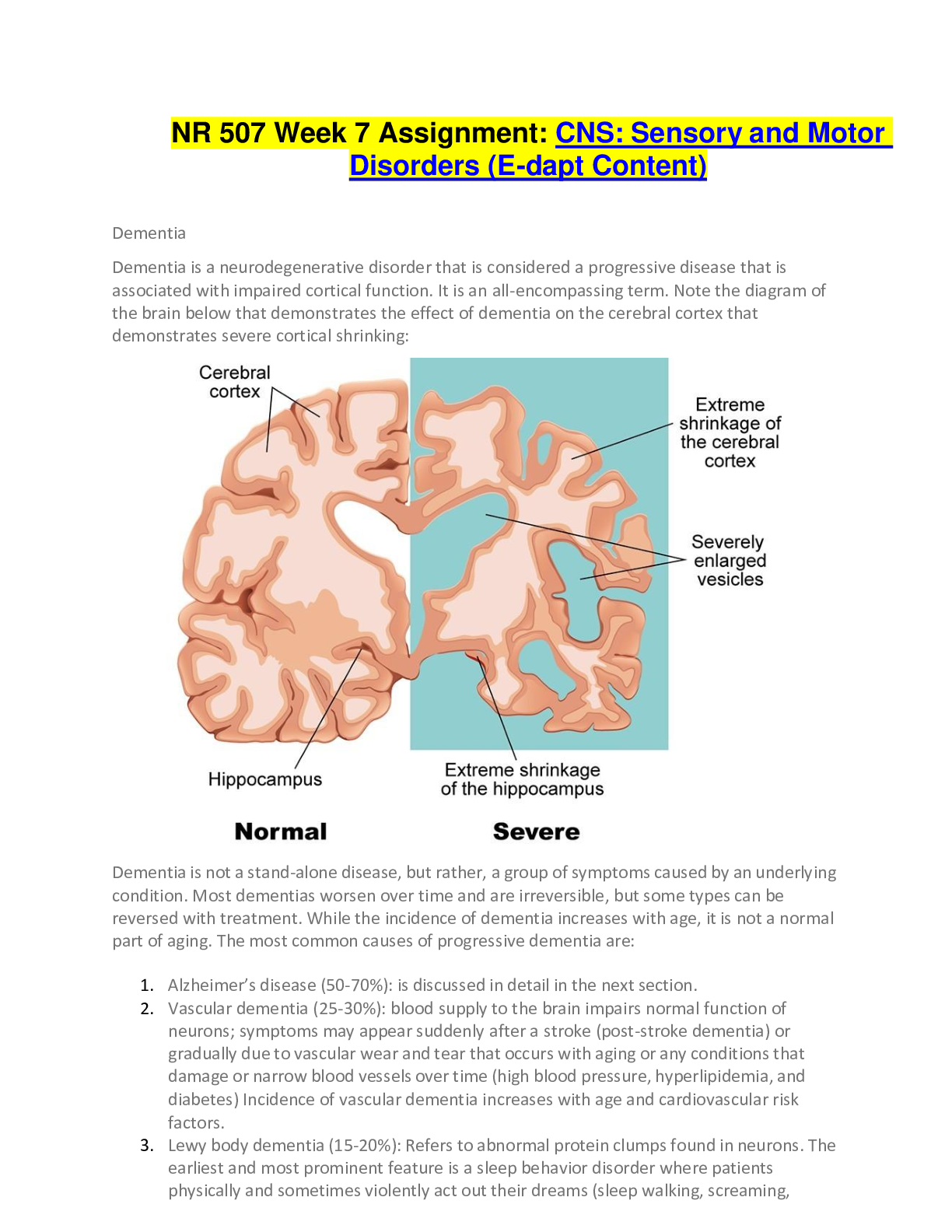
NR 507 Week 7 Assignment: CNS: Sensory and Motor Disorders (E-dapt Content) Dementia Dementia is a neurodegenerative disorder that is considered a progressive disease that is associated with impai... red cortical function. It is an all-encompassing term. Note the diagram of the brain below that demonstrates the effect of dementia on the cerebral cortex that demonstrates severe cortical shrinking: Dementia is not a stand-alone disease, but rather, a group of symptoms caused by an underlying condition. Most dementias worsen over time and are irreversible, but some types can be reversed with treatment. While the incidence of dementia increases with age, it is not a normal part of aging. The most common causes of progressive dementia are: 1. Alzheimer’s disease (50-70%): is discussed in detail in the next section. 2. Vascular dementia (25-30%): blood supply to the brain impairs normal function of neurons; symptoms may appear suddenly after a stroke (post-stroke dementia) or gradually due to vascular wear and tear that occurs with aging or any conditions that damage or narrow blood vessels over time (high blood pressure, hyperlipidemia, and diabetes) Incidence of vascular dementia increases with age and cardiovascular risk factors. 3. Lewy body dementia (15-20%): Refers to abnormal protein clumps found in neurons. The earliest and most prominent feature is a sleep behavior disorder where patients physically and sometimes violently act out their dreams (sleep walking, screaming, laughing, kicking, punching). It is a REM sleep disorder with failure to inhibit movement during sleep. There can also be visual hallucinations; memory loss may not be noticeable until later stages. Dementia caused by advanced Parkinson’s disease dementia belongs in this group and will be discussed later in more detail. 4. Frontotemporal dementia: is another common type of progressive dementia. It is characterized by neuronal cell death in the frontal and temporal lobes of the brain. The areas associated with this relates to behaviors and language. Common signs and symptoms include changes in behaviors (social inappropriateness, impulsivity, apathy, emotional indifference) and language deficits This type has a strong genetic component and tends to occur earlier between the ages of 40-50 years. More than one type of the dementias mentioned above may co-exist in one patient (mixed dementia). Less common causes of dementia include Huntington’s disease, Creutzfeldt-Jakob disease and traumatic brain injuries. Dementias may also develop from metabolic and endocrine processes such as thyroid disorders and vitamin deficiencies (B1, B6, B12) or infections like Lyme disease and neurosyphilis. For these types of dementias, symptoms can be reversed. Alzheimer’s Disease Alzheimer’s disease is the most common neurogenerative disease in the world. Ten percent of individuals over the age of 65 have Alzheimer’s disease. Alzheimer’s, like dementia, is a progressive disorder associated with impaired cognitive function and impaired cortical skills. The speed by which it develops varies among individuals and can occur over many years (4, 8 or 20 years). It is associated with neuronal loss (dying or atrophy of the neurons). This results in a smaller brain. In the brain of a patient with Alzheimer’s disease as compared with the normal brain in the diagram below, there is cortical atrophy and overall reduction in brain size and weight which causes an overall reduction in cortical mass. There is also enlargement of the ventricles and reduction in size of the hippocampus. The signs and symptoms associated with Alzheimer’s disease is based on the changes in the brain. A reduced hippocampus size is associated the decreased short-term memory, but the individual’s long-term memory may be intact. Because Alzheimer’s disease is a progressive, it spreads from the hippocampus to more cortical regions of the brain. At that point, the long- term memory can become altered. The Cause of Alzheimer’s Disease The cause of Alzheimer’s disease is unknown. There are some underlying genetic and environmental causes, though, that interact to cause the disease. There are several hypotheses that explain the development of Alzheimer’s disease. • • Amyloid Hypothesis • Cholinergic Hypothesis • Glutamate Excitotoxicity In the healthy brain, the Tau protein helps to lengthen and support the microtubule structure. Microtubules play a crucial role in the transport of nutrients and information molecules throughout the neuron. When Tau dissociates, the microtubule assembly becomes compromised thereby disrupting the neuron’s transport system leading to malfunctions in biochemical communication between neurons. Neurofibrillary tangles are involved in this process. Remember that healthy brain neurons will send signals from the cell body down the axons to the synapse so that it can release neurotransmitters. In addition to sending action potentials, neurons also send proteins, nutrients and other important molecules. The microtubules are responsible for carrying these. In Alzheimer’s disease, the microtubules become misfolded and tangled which alters their function. These are called neurofibrillary tangles. Amyloid hypothesis This hypothesis involves the formation of senile plaques (amyloid plaques). Beta-amyloid is a metabolic waste disorder found in the fluid between the brain cells. It induces neuroinflammation and disrupts communication between the neurons. On the plasma membrane, there are numerous proteins. Some proteins ensure appropriate neurotransmitter function at the synapse. Other proteins, like the neurofibrillary tangles also misfold, malfunction and then get released into the extracellular fluid and come together to form an even larger misfolded protein (Beta-amyloid clump). This is a senile plaque. The diagram to the right depicts neurofibrillary tangles. Cholinergic hypothesis This hypothesis involves reduced levels of acetylcholine (ACH). ACH, is a cholinergic, excitatory neurotransmitter in the central nervous system. It is important in forming new neuronal function and synapses that involve memory. It is unclear why ACH is reduced in Alzheimer’s disease. But it provides us an opportunity manage the signs and symptoms pharmacologically. If there is reduced ACH, it needs to be increased. One of the ways to increase ACH is by reducing the amount of enzyme that breaks down ACH. The enzyme is called acetylcholine esterase (ACH esterase). If a patient can receive an ACH esterase inhibitor, it will stop the enzyme from destroying ACH and improve ACH levels in the system. Note that there is no cure for Alzheimer’s disease and the neurodegenerative process cannot be stopped, but if ACH can be increased for as long as possible, then some symptoms can be controlled. Glutamate exotoxicity Another theory on what causes Alzheimer’s disease involves glutamate excitotoxicity. Glutamate is another excitatory neurotransmitter of the CNS. It works on the neuron. If we have a neuron and we want that neuron to fire off an action potential, starting at the cell body and send it to the neurotransmitters, we need to excite it. Glutamate can be given. Glutamate comes down to the cell body to bind with glutamate receptors. Once this occurs, the action potential is sent. Remember that neurons are negative on the inside of the cells. Positive ions located on the outside rush into the cell in a domino effect. Glutamate stimulates this by binding to receptors to open the calcium channels that allows calcium to rush inside the cell. When the positive ion Calcium enters the cell, it will lead to depolarization and production of an action potential. In Alzheimer’s disease, the patient may have too much glutamate being released which results in a toxic effect. If too much glutamate binds with the neuron, then too much calcium is going into the cell. Too much calcium entering a cell usually results in cell death. This is one thought on how cells begin to die off in Alzheimer’s disease. The diagram below depicts the result of too much calcium ion entering the cell with the increase in free radical, degradation of the cell membrane and proteins. Stages of Alzheimer’s Disease Because of the varied progressive nature of Alzheimer’s disease, it is difficult to describe distinct stages. The stages also overlap. Keeping this in mind, the stages are described below. Note that before we review the stages of Alzheimer’s disease below, it is important to recognize that to actually diagnose it, other potential causes of dementia should be ruled out including the effects of medication, trauma, vascular issues such as stroke and ischemia first. Once ruled out, it can be a presumptive diagnosis based on MRI results that demonstrate atrophy in the hippocampus. Stage 3: Mild Cognitive Impairment Where There are Signs of Early Confusion Subtle difficulties can begin to impact the individual’s daily life. The individual may consciously or subconsciously attempt to hide the issues. There may also be difficulty in retrieving words, remembering what was just read. These can affect home life and work. It can still be difficult to diagnose Alzheimer’s at this early stage. Stage 4: Mild Alzheimer’s Disease This stage will last for approximately two years. During this time, it may be difficult to manage finances and engage in activities that involve math. It will also be difficult to remember recent events and things that were recently learned. The individual may have difficulty completing tasks, especially if there are specific sequences involved in completing the task such as cooking or driving. Individuals are still able to recognize their family and friends. A diagnosis at this stage is usually accurate. Stage 5: Early Dementia to Early Alzheimer’s Disease In this stage, cognitive decline becomes more pronounced. The patient requires assistance. The individual will have difficulty remembering things like address or phone number. The individual can also become disoriented very easily regarding time or place. Decisions and judgement skills are also affected. They may not be able to choose the appropriate clothes for day or season. The individual may need increased supervision. This stage can last on average 1.5 years. Stage 6: Moderately Severe Alzheimer’s Disease There is a significant lack of awareness of present events. The inability to remember the past and carry on a conversation may occur. The individual will need help with basic activities of daily living and are unlikely to be able to recall names of family members but will recognize them as familiar. This stage lasts approximately 2.5 years. Stage 7: Severe Alzheimer’s disease In this stage, speech becomes severely limited. There is a very serious decline in basic abilities. Even movement abilities become affected as the disease has begun to spread to those areas of the brain. Eating, walking, sitting or standing become very difficult to the point where the individual requires assistance. Assistance is also need for eating and drinking due to loss of the ability to discern thirst or hunger. Total assistance is needed around the clock. Due to these inabilities, the individual become more susceptible to secondary diseases such as pneumonia, infection, and falls. This stage can last from 1-2 years. It is important to stress the flexibility of these timelines within these stages. Not all patients with Alzheimer's disease will experience the exact progression. Some may deviate significantly from the expected duration of each stage and the disease itself. Diagnosis of Alzheimer’s Disease There is no one test that will show if someone has dementia or Alzheimer’s disease since the typical symptoms like memory loss, confusion, and others can have many possible causes. It is not completely diagnosed until a complete medication assessment is performed and other causes of dementia ruled (medication, trauma, vascular issues). Once those are ruled out, MRI may reveal hippocampus atrophy that can point to Alzheimer's type dementia. Dx test: Urine and blood evaluation: This information can help determine if there are any other health issues that are causing or contributing to Alzheimer’s symptoms such as heart problems and vascular dementia. Other issues can also be confirmed or ruled out that include anemia, depression, infection, diabetes, kidney disease and others that can cause dementia-like symptoms like confused thinking and memory problems. Motor and sensory function should be tested along with deep tendon reflexes. Neuropsychological tests to objectively measure the patient’s current memory and mental abilities can be performed: o Mini-mental state examination (MMSE): this helps in diagnosing dementia because it looks at orientation, memory and attention. The patient is asked to follow verbal or written commands or write a sentence spontaneously or copy a complex shape. Depending on the score, a certain level of dementia might be suggested. The rating scale is below: o 20-24: Mild dementia o 13-20: Moderate dementia o < 13: Severe dementia o Patients with Alzheimer’s disease tend to drop 2-4 points on average/year. o Mini-Cog: o Name 3 objects and repeat them back to the practitioner. o Next draw a clock and designate a specific time. o Then the patient is asked to repeat the original 3 objects. If the patient fails one or all the tasks, it is suggestive of dementia where further evaluation is recommended. This is a mood assessment where the patient’s current level of well-being is assessed. The purpose it to determine signs of depression and other mood disorders that can contribute to symptoms that overlap with dementia. o Brain Scans: o Computed tomography (CT) o Magnetic resonance imaging (MRI) o These may be useful in identifying larger masses such as tumors that contributes to cognitive impairment. It may also help in making a differential diagnosis where the NP attempts to determine the type of dementia. By looking at where most atrophy of brain tissue occurs can help determine the type of dementia: o Hippocampus: is suggestive of Alzheimer’s disease o Frontal lobe: is suggestive of frontotemporal dementia o Vascular pathologies suggest vascular dementia Treatment of Alzheimer’s Disease In treating Alzheimer’s disease, current therapeutic options are limited to drugs that only provide mild symptomatic benefit. The drugs are divided into two classifications: Cholinesterase Inhibitors Under normal conditions, cholinergic neurons in the brain synthesize acetylcholine from acetyl coenzyme A (acetyl CoA) and choline, in a reaction catalyzed by an enzyme choline acetyltransferase. Upon arrival of neuronal impulses, ACH enters the synaptic cleft where it interacts with ACH receptors located on the post-synaptic neuron. Shortly afterwards, an enzyme, acetyl cholinergic esterase ACHL and butyryl cholinesterase (BuChE), break down acetylcholine into acetate and choline that terminate stimulating signals. Since Alzheimer’s disease has been linked to a deficiency of ACH in the brain, cholinesterase inhibitors are introduced to alleviate the symptoms. They work by inhibiting cholinesterase enzyme from breaking down ACH by increasing both the level and duration of the action of ACH. The three commonly prescribed cholinesterase inhibitors are: • Donepezil (Aricept) • Rivastigmine (Exelon) • Galantamine (Reminyl) It is important to note that out of the three medications, rivastigmine (Exelon) is the only one that shows significant inhibition of both acetylcholine esterase and butyryl cholinesterase. Side effects can range from mild (nausea, vomiting, diarrhea) to potentially serious (bradycardia, decreased appetite, and substantial weight loss). NMDA Receptor Antagonists NMDA receptors belong to a family of ionotropic glutamate receptors that mediate most of the excitatory synapse transmission in the brain. They are thought to play an important role in learning and remembering information. Beta amyloid proteins accumulate in the brain of Alzheimer’s patients and may cause an abnormal rise in extra synaptic glutamate levels by inhibiting glutamate uptake or triggering glutamate release from the glial cells. The binding of glutamate to the NMDA receptor results in the influx of extracellular calcium which controls membrane excitability and synaptic transmission. When glutamate levels become abnormally elevated, overstimulation of NMDA receptors can result, leading to excessive influx of calcium ultimately causing cell rupture and death. Memantine is an NMDA receptor antagonist that works by blocking NMDA receptors which limits the influx of calcium into the neuron. Common side effects are diarrhea, headache and insomnia. Parkinson’s Disease Parkinson’s disease (PD) is a movement disorder where the dopamine-producing neurons and substantia nigra of the brain undergo neuron degeneration. It is one of the most common neurological disorders. It is progressive with its onset during adulthood. Therefore, it is more common with age. Most of the time, there is no known cause. In some cases, however, there may be a genetic cause. In this case, there are mutations in the PINK1, Parkin, or Alpha synuclein genes. In rare cases, Parkinsonian symptoms may be caused by MPTP, a toxic impurity that is found in the recreational drug MPPP (synthetic opioid). In other individuals, one or more risk factors rather than a single cause may contribute to Parkinson’s disease. This includes pesticide exposure or DNA variance in genes like LRRK2. Regardless of the cause, PD is derived from the death of dopamine-producing (dopaminergic) neurons in the substantia nigra. The name substantia nigra means “black substance”. It is darker than other brain regions when viewing a slice of the brain on autopsy. There are two regions of the substantia nigra. There is one located on each side of the mid-brain. It is part of the basal ganglia, a collection of brain regions that controls movements through their connections to the motor cortex. In PD, the darkened areas of the substantia nigra gradually disappear. Under a microscope, Lewy bodies which are eosinophilic, round occlusions made up of alpha-synuclein protein are present in the affected substantia nigra neurons before they die. The function of alpha-synuclein is unknown as well as the significance of Lewy bodies. They are also both found in other diseases like Lewy body dementia and multiple-system atrophy. The substantia nigra can be split into two subregions: 1. Pars reticulata: It receives signals from another part of the basal ganglia called the striatum. It relays messages to the thalamus via by the neurotransmitter Gamma amino butyric acid (GABA). 2. Pars compacta: This is the part of the substantia nigra that is affected in PD. It sends messages to the striatum via neurotransmitter dopamine via the nigrostriatal pathway which helps to stimulate the cerebral cortex to initiate movement. Therefore, when substantia nigra pars compacta neurons die, the individual might be in a hypokinetic, low movement state which is commonly seen in PD. In addition to simply initiating movements, the substantia nigra helps to calibrate and fine-tune the way that movements happen. This leads to the clinical features of PD. Clinical Manifestations of Parkinson’s Disease Tremor: This is involuntary shakiness most noted in the hands (pill-rolling movement). This is called a resting tremor indicating that it is present at rest. It diminishes with intentional movement. Rigidity: This is stiffness that can appear as a cogwheel rigidity. This is a series of catches or stalls in movement as the extremity is passively moved by someone else. Rigidity is also responsible for stooped posture and almost expressionless face. Bradykinesia: This is slow movement. Hypokinesia is a lessened movement and akinesia is no movement. All three result from difficulty initiating movement. Examples include having the legs freeze up when attempting to walk and walking with a shuffling gate or small steps. Postural instability: This is a late feature of PD. It causes problems with balance and can lead to falls. Despite these multiple effects in movement, PD does not produce weakness. This helps to differentiate it from diseases that affect the motor cortex or corticospinal pathway diseases. Also, the resting tremor of PD helps to differentiate it from cerebellar diseases which may result in an action or intention tremor. This is the opposite of a resting tremor where the tremor actually worsens with movement. Both bradykinesia and postural instability help differentiate PD from essential tremor. It is an action tremor which is also a hallmark feature Non-motor brain functions can also be affected by PD: • Depression • Dementia • Sleep disturbances • Difficulty smelling These are thought to arise due to dysfunction of dopaminergic signaling in other parts of the brain beyond the substantia nigra. For example, in the pre-frontal cortex, it can lead to cognitive symptoms. Treatment of Parkinson’s Disease Treatment of PD does not stop the progression of the disease, but it does help to control the symptoms. 1. The main strategy of treatment is to increase the amount of dopamine signaling in the brain. Dopamine itself cannot cross the blood-brain barrier. But its precursor Levodopa can. Once in the brain, levodopa is converted to dopamine by dopa decarboxylate in the remaining nigrostriatal neurons. Peripheral dopa decarboxylase also exists which can metabolize levodopa before it gets to the blood-brain barrier and via additional enzymes, metabolize it into other catecholamines like epinephrine. This is the reason that levodopa is administered with carbidopa a dopa decarboxylase inhibitor that is not able to cross the blood-brain barrier. 1. The next strategy is to use amantadine (Gocovri)This is an antiviral medication that increases endogenous dopamine production although, the mechanism is not fully understood. 2. Dopamine agonists: These stimulate dopamine receptors to trick the brain in thinking that there is more dopamine than exists (Bromocriptine (Cycloset), Pramipexole (Mirapex), Ropinirole (Requip XL) 3. Inhibitors of COMT: Catecholamine –O-methyltransferase-it is an enzyme that degrades dopamine and levodopa. These are inhibitors that are given with levodopa to prevent the enzyme from breaking it down outside the CNS. This allows more of it to enter the brain. In a similar fashion, there are medications like selegiline (Eldepryl) that inhibits monoamine oxidase-B (MAO-B) which is another enzyme that breaks down dopamine. A special treatment available to help treat PD is deep brain stimulation. It involves an implantable device that directly sends electrical signals to the basal ganglia which counteracts the aberrant signaling in PD. Multiple Sclerosis Multiple sclerosis (MS) is a demyelinating disease of the central nervous system which includes the brain and the spinal cord. Myelin is the protective sheath that surrounds the axon of neurons that allows them to quickly send electrical impulses. The myelin is produced by oligodendrocytes which are groups of cells that support neurons. In MS, demyelination happens when the immune system inappropriately attacks and destroys the myelin which breaks down communication between the neurons. Ultimately it results in sensory, motor, and cognitive problems. The brain and its neurons are protected by the blood-brain barrier. It only allows certain molecules and cells to pass through from the blood. For immune cells like T and B cells, that means that they must have the right ligand or surface molecule to get through. Once a T-cell makes its way into the brain, it can get activated by something that it encounters. In the case of MS, it is activated by myelin. Once activated, the T-cell changes the blood brain barrier to express more receptors that allows immune cells to more easily bind the receptors to gain entry. MS is a type IV hypersensitivity reaction (cell-mediated). This means that the myelin-specific T- cells release cytokines (Interleukin-1, interleukin-6, tumor necrosis factor-alpha, interferon- gamma). Together, these dilate the blood vessels which allows for more immune cells to gain access as well as directly cause damage to the oligodendrocytes. The cytokines also attract B- cells and macrophages as part of the inflammatory process. The B-cells start to make antibodies that mark the myelin sheath proteins. Then the macrophages use those antibodies as markers to engulf and destroy the oligodendrocytes. Without oligodendrocytes, there is no myelin to cover the neurons. This leaves behind areas of scar tissue also known as plaques or sclera. In MS, the autoimmune system attacks happen in bouts. In other words, an autoimmune attack on oligodendrocytes might happen and then regulatory T-cells will come in to inhibit or calm down the other immune cells, leading to a reduction in the inflammation. In early MS, the oligodendrocytes will heal and extend out new myelin to cover the neurons. This is called remyelination. Unfortunately, over time as the oligodendrocytes die off, the remyelination stops. The damage becomes irreversible with the loss of axons. Types of Multiple Sclerosis There are four types of MS that are based on the patterns of symptoms over time. Use the following diagram as a reference for understanding the four types: Clinical Manifestations of Multiple Sclerosis Symptoms vary from person to person and depend largely on the location of the plaques. It affects individuals between the ages of 20-40 years. Symptoms related to bouts can typically worsen over a week and linger for months without treatment. One common trio of MS symptoms is Charcot’s Neurologic Triad: Dysarthria This is difficulty or unclear speech that is due to plaques in the brain stem. They affect nerve fibers that control muscles of the mouth and throat that can interfere with conscious movements like eating and talking; it can also affect unconscious movements such as swallowing. Nystagmus This is involuntary rapid eye movement due to plaques on the nerves that cause eye movements. Plaques on the optic nerve causes loss of vision in one or both eyes (optic neuritis). In addition, if there is damage to the nerve controlling eye movement, eye movements can be painful. There can also be double vision if the eyes no longer move in an uncoordinated way. Intention Tremor This can be caused by plaques along the motor pathways of the spinal cord which can affect outbound signals and skeletal muscle control. Motor symptoms include muscle weakness and spasms, tremors and ataxia which is a loss of balance and coordination. In serious cases, this can lead to paralysis. Plaques located in the sensory pathways can affect inbound signals such as sensations from the skin, which cause sensations like numbness, pins and needles and paresthesia. Sometimes there can be very specific sensory sensations as in the case of Lhermitte’s sign. It is when an electric shock runs down the back and radiates in the limbs when bending the neck forward. Plaques can also involve the autonomic nervous system. It can lead to bowel and bladder symptoms like constipation and urinary incontinence. Sexual dysfunction can also occur. Finally, MS can also affect higher order activities of the brain causing poor concentration and critical thinking as well as depression and anxiety. Multiple Sclerosis Diagnosis and Treatment Diagnosis MS is suspected when there are multiple neurological symptoms separated in space which is attributed to damage in different locations in the nervous system as well as time. It is characterized by periods of exacerbations and remissions. The diagnosis of MS is supported by: 1. An MRI that shows multiple CNS lesions called white matter plaque. 2. Cerebrospinal fluid that has the presence of high levels of antibodies indicating an autoimmune process. 3. Visual evoked potential that measures the nervous system’s response to visual stimuli. Treatment There is no cure for MS, but there are medications that are effective for the relapsing, remitting type. Medications can lessen the severity of relapses and help them happen less frequently. 1. Corticosteroids 2. Cyclophosphamide: A cell cycle inhibitor 3. Intravenous immunoglobulin All of these can be used to block the autoimmune process. In addition, plasmapheresis can be effective where the plasma is filtered to remove disease-causing autoantibodies. Chronic treatment of MS includes immunosuppressants like recombinant beta- interferon that decreases the level of inflammatory cytokines in the brain as well as increases the function of T- regulatory cells. Other immunosuppressants block T-cells from getting into the brain by interfering with the cell surface molecule used to gain passage through the blood-brain barrier. Unfortunately, though there are fewer treatment options available for progressive MS. Instead, treatments are often aimed at managing specific symptoms. Finally, physical therapy and cognitive rehabilitation therapy can be particularly helpful with sensory, motor and cognitive symptoms Drugs used to treat restless leg syndrome include: • Dopamine drugs. • Benzodiazepines. • Opioids. • Anticonvulsants. • Alpha-2 agonists. • Iron. Restless Leg Syndrome/Periodic Limb Movement Disorder Restless leg syndrome (RLS) occurs when going to sleep and progresses over time. The individual has the urge to move legs and experience an uncomfortable sensation in their legs. Symptoms are improved with movement and worsen with relaxation and occurs later in the day. Unlike RLS, periodic limb movement disorder (PLMD) occurs during sleep and involves involuntary cyclic movements of the lower extremities. The movements typically occur with flexion of the great toe accompanied by partial flexion of the ankle, knee and sometimes the hip. There must be four movements in a row to be PLMD with 15 PLMD per hour present to be diagnosed as PLMD (approximately every 20-40 seconds). There is also an association between periodic limb movement disorder (PLMD) and RLS. Up to 85% of individuals with RLS also have PLMD. If the individual has RLS and PLMD together, it may be lumped into the category of RLS and not be diagnosed at the same time. RLS and PLMD share a similar pathophysiology where the exact mechanisms are not completely understood. There seems to be a defect in the regulation of iron that leads to dysfunction in the dopamine system. Although RLS and PLMD share a similar pathophysiology, diagnosis of the two condition is different. RLS can be diagnosed based on clinical criteria alone. A polysomnography (PSG) may sometimes be used to rule out other sleep disorders. To diagnose PLMD, a sleep study with limb electrode measurement is required. Finally, patients with RLS or PLMD should have a serum ferritin measured to rule out an iron- deficiency that leads to the clinical manifestations of RLS or PLMD. Diagnosis of Restless Leg Syndrome The DSM-5 criteria may be used to diagnose RLS as there is a psychological component to the disease. It is important to note that it is a patient-reported disease. Therefore, there is no specific lab that diagnoses it. From a diagnosis standpoint, the individual must: • Have an urge to move the legs usually followed by discomfort if the person does not move the legs. • It is worse at night and when the individual is inactive. The symptoms improve with movement. • It gets better with movement • Can’t be due to another cause such as intoxication or medication. Clinical Manifestations of Restless Leg Syndrome Below are a few clinical manifestations of restless leg syndrome. Treatment for Restless Leg Syndrome Non-Pharmacological • Exercise is encouraged to help with discomfort. • If the person is taking an SSRI, it should be discontinued due to the side effects of RLS. • Avoid caffeine, alcohol, and nicotine. Pharmacological The treatment for RLS and PLMD is similar. However, for PLMD, the decision to initiate treatment is controversial. For both RLS and PLMD, the treatment must be individualized based on the severity of the symptoms and its impact on the patient’s quality of life. The medication recommendations below are based on the American Academy of Neurology 2016 and the American Academy of Sleep Medicine 2012 guidelines. Note that the mechanism of action of these drugs are not completely understood in symptom relief of RLS or PLMD but are thought to act on the dopaminergic projects descending from the A11 region of the hypothalamus to the spinal neuronal systems. It is dysfunction here that causes the symptoms. On the somatic side of the spinal cord there are sites for the effects of dopamine, adenosine A1, adenosine A2 and opioid receptor in the dorsal horn that help to alleviate the symptoms. On the autonomic side, actions of dopamine and adenosine receptors have been identified on sympathetic preganglionics with evidence of opioid action here as well. Alpha-2 agonists modifies the excitability of the target tissues by directly binding to calcium channels. Class Generic Dopaminergic agents Ropinirole Pramipexole Carbidopa/levodopa Alpha-2 agonists Gabapentin Pregabalin L Benzodiazepines Clonazepam Temazepam Opioid agents Codeine Tramadol Oxycodone Hydrocodone Methadone In addition to the pharmacologic agents above, iron supplementation should be prescribed if the serum ferritin is less than 50-70 ng/dL. Neuropathies Peripheral neuropathy has multiple etiologies but there are only a few ways, however, that nerves can respond to an injury. The damage can occur at: 1. Level of the axon (axonopathy). 2. Distal to the site of injury (Wallerian degeneration). 3. Distal portion of the axon (degeneration of axon and myelin sheath). Myelopathies occur at the level of the myelin sheath due to heredity or inflammation. In hereditary demyelinating neuropathies the axons are spared. Therefore, complete recovery can occur in a few weeks to months. This type of neuropathy is characterized by patchy, segmental injuries. Neuropathies can be examined based on their pattern of involvement: focal, multifocal, or symmetric. A focal neuropathy is a chronic condition with sensory, motor, or mixed involvement. It involves microvascular compression that results in neural ischemia, paresthesia, intraneural edema that leads to more microvascular compression. Demyelination occurs that results in fibrosis and loss of axon. The first sensations lost include light touch, pressure and vibration followed by later loss of pain and temperature sensation. The most common compressive neuropathy in the upper extremity is carpal tunnel syndrome. The diagram below shows compression of the median nerve at the wrist that results in pain and numbness. Transcript Link Multifocal A multi-focus neuropathy is purely a motor neuropathy that is characterized by motor deficits in the distribution of single nerve without associated sensory loss caused by vasculitis or diabetes. This type is relatively uncommon and there is always asymmetric involvement typically in the upper limbs. Weakness begins in the distal upper limbs that causes difficulty in wrist and finger extension or reduced hand grip. Symmetric In a symmetric neuropathy, it is important to determine if it is proximal or distal. Motor neuropathies are predominantly proximal (Guillain-Barre syndrome). Proximal sensory neuropathies are rare (porphyria). In distal symmetric neuropathy, this is seen with most metabolic, toxic and nutritional issues. It is predominantly in the axon and is length dependent. Symptoms begin in the feet and progress with the characteristic stocking glove pattern. Dysfunction first occurs in the distal portions of the longest axons, thus in the most distal portions of the extremities. Small diameter axons are most susceptible to metabolic injury due to their small size. Initial symptoms include autonomic dysfunction and small fiber sensory modalities, including loss of pain and temperature. Patients will present with numbness, paresthesia, burning discomfort, with loss of sensation in the distal lower extremity. Later there will be loss of an Achilles tendon reflex and weakness of the intrinsic foot muscles. Neuropathic Patterns of Select Neuropathies Review the different neruopathic patterns of select neuropathies below. Distal symmetric diabetic neuropathy consists of two sub-types: • Large-fiber (A-type): This involves deep-seated pain (A-type); signs and symptoms include wasting and weakness; numbness, pins and needles, tingling, ataxia, impaired vibration perception; loss of position sense; loss of reflexes; impaired nerve conduction velocity; it interferes with normal life and there is a risk of falls and fractures. • Small-fiber: Involves superficial pain (C-fiber type); will feel electric shock, burning, allodynia Autonomic dysfunction, thermal imperceptions; normal strength and reflexes; quantitative sensory testing and skin biopsies produces symptoms and leads to morbidity and mortality. Toxic neuropathy One of the most common causes of toxic neuropathy is from complications of cancer treatment following the administration of chemotherapy. Chemotherapeutic drugs commonly associated with toxic neuropathy include platinum-derived, taxane, and vinca alkaloid compounds. High levels of these drugs are found in the dorsal root ganglia and peripheral nerves. Reversibility of the signs and symptoms following discontinuation of the drugs vary: Other drugs which can induce neuropathy includes: 1. Antimicrobials: e.g. Chloroquine, nitrofurantoin. 2. Cardiovascular: Amiodarone, digoxin, statins. 3. Immunosuppressants: Interferon. 4. Miscellaneous: Cimetidine, colchicine, levodopa, lithium and phenytoin. There are some neuropathies that present with an uncommon pattern: 1. Neuropathies that involve the cranial nerves as in the case with Guillain-Barre syndrome that can involve the facial nerves. 2. Greater involvement of the arms than the legs. This is the case with leprosy that tends to involve cutaneous nerves in the cooler areas of the body (tip of the nose). Neuropathies can also be grouped according to the fiber type that is mostly involved: 1. Toxic and metabolic neuropathies are sensory initially, but later involve the motor fibers. 2. Total sensory neuropathies may result from drug toxicity (cisplatin), nutritional deficiencies, and paraneoplastic syndromes. 3. Total motor neuropathies include Guillain-Barre syndrome. 4. Painful neuropathies include alcoholism and diabetes due to small-fiber involvement. It is also important to determine if the neuropathy is axonal, demyelinating or both. The best method for determining this is through nerve conduction studies (NCS) and electromyography (EMG). Damage occurs in the small fibers of the peripheral nervous system. Several diseases may lead to small-fiber neuropathy and include: • Metabolic syndrome and impaired glucose tolerance. • Vitamin B-12 deficiency. • Thyroid dysfunction. • Neurotoxic medications. • Sarcoidosis. • HIV. • Paraneoplastic syndromes. • Celiac disease. • Paraproteinemia. There are some neuropathies that present with an uncommon pattern: 1. Neuropathies that involve the cranial nerves as in the case with Guillain-Barre syndrome that can involve the facial nerves. 2. Greater involvement of the arms than the legs. This is the case with leprosy that tends to involve cutaneous nerves in the cooler areas of the body (tip of the nose). Neuropathies can also be grouped according to the fiber type that is mostly involved: 1. Toxic and metabolic neuropathies are sensory initially, but later involve the motor fibers. 2. Total sensory neuropathies may result from drug toxicity (cisplatin), nutritional deficiencies, and paraneoplastic syndromes. 3. Total motor neuropathies include Guillain-Barre syndrome. 4. Painful neuropathies include alcoholism and diabetes due to small-fiber involvement. It is also important to determine if the neuropathy is axonal, demyelinating or both. The best method for determining this is through nerve conduction studies (NCS) and electromyography (EMG). Autonomic neuropathy Autonomic neuropathy involves damage to the nerves that regulate body functions that is out of the individual’s control. It includes regulation of heart rate, blood pressure, perspiration and digestion. One of the best examples to illustrate autonomic neuropathy is reviewing the signs of autonomic diabetic neuropathy summarized in the diagram below. It shows the extent of body systems involved along with associated responses and treatments: System Symptoms Treatment Cardiovascular Resting tachycardia, reduced exercise tolerance, orthostatic hypotension, asymptomatic myocardial ischemia Symptomatic treatment including ACE-in clonidine, graded exercise programs, lifes cardiovascular comorbidity Gastrointestinal Esophageal dysmobility, gastroparesis, Prokinetic agents, anti-diarrheals (e.g. lop diarrhea, bacterial overgrowth Genitourinary Erectile dysfunction, cystopathy, female sexual PDE5 inhibitors (e.g. sildenafil), psycholog dysfunction prostacyclin lubricants, intermittent cathe Metabolic Hypoglycemic awareness, autonomic failure Optimal glycemic control Sudomotor Anhidrosis, heat intolerance, gustatory sweating, dry skin Emollients, vasodilatiors, glycopyrrolate, Legend: ACE – angiotension-converting enzyme, PDE5 – phosphodiesterase 5. There are some neuropathies that present with an uncommon pattern: 1. Neuropathies that involve the cranial nerves as in the case with Guillain-Barre syndrome that can involve the facial nerves. 2. Greater involvement of the arms than the legs. This is the case with leprosy that tends to involve cutaneous nerves in the cooler areas of the body (tip of the nose). Neuropathies can also be grouped according to the fiber type that is mostly involved: 1. Toxic and metabolic neuropathies are sensory initially, but later involve the motor fibers. 2. Total sensory neuropathies may result from drug toxicity (cisplatin), nutritional deficiencies, and paraneoplastic syndromes. 3. Total motor neuropathies include Guillain-Barre syndrome. 4. Painful neuropathies include alcoholism and diabetes due to small-fiber involvement. It is also important to determine if the neuropathy is axonal, demyelinating or both. The best method for determining this is through nerve conduction studies (NCS) and electromyography (EMG). Clinical Manifestations of Neuropathy The clinical manifestations and overall course of the neuropathy depends on its etiology. If the neuropathy is caused by trauma or ischemia, it will have an acute onset and will demonstrate the most severe symptoms at that time. Neuropathies with a metabolic or inflammatory etiology will have a subacute course that may last from days to weeks. Characteristically, toxic and metabolic neuropathies are chronic, lasting from weeks to months. Inherited neuropathies are chronic and slowly progressing. Finally, some neuropathies have a relapsing and remitting course as in the case of Guillain-Barre syndrome. The practitioner can examine the symptoms closely to determine the type of axons involved: 1. Ischemic neuropathies: Pain is the most prominent feature. 2. Small fiber neuropathies present with burning, lightening, or aching pain or paresthesia; may be intolerant to sensations. e.g. when a sheet touches their feet (allodynia); may also described band-like sensation around ankles or wrists. 3. Sensory symptoms: Tingling, paresthesia, hypesthesia, numbness. 4. Distal symmetric axonal neuropathies initially involve the tips of toes and progress progressively in a stocking-glove distribution. 5. Multifocal neuropathies: Mononeuritis multiplex that is caused by polyarteritis nodosa. 6. Can present as restless leg syndrome. 7. Proximal involvement: May involve difficulty climbing stairs, getting out of a chair, lifting and swallowing. Neuropathy Diagnosis and Treatment Diagnosis The practitioner must collect a thorough health history specifically determining if the neuropathy can be associated with a systemic disease (diabetes, hypothyroidism). Medication history should be collected to determine if there are neuropathic side effects. A social history should be collected to identify drug and alcohol use. All patients should be assessed for HIV risk factors, foreign travel (leprosy), diet, vitamin use (vitamin B-12) and any history of tick bite (Lyme disease). Family history should be obtained to identify any hereditary factors that may contribute to neuropathy. A complete review of systems will help to determine any organ involvement and any malignancy. Diagnostic Tests EMG and nerve conduction studies (NCS) are the most useful diagnostic tests to use when evaluating peripheral neuropathy. They can determine the presence of the neuropathy as well as identify the type (motor or sensory), the pathology (axonal loss or demyelination) and whether it has a symmetric, asymmetric or multifocal pattern of involvement. Treatment Treatment involves eliminating the cause of the neuropathy if at all possible. If is it caused by an associated disease such as diabetes, then the disease should be controlled. Care should be taken to avoid further damage to the axon by avoiding trauma, accident and hypoxia and ischemia. Pharmacological management includes medications to treat associated diseases and treating neuropathic pain. Some of the medications used are listed below: Medication Effects Tricyclic antidepressants (amitriptyline, nortriptyline) Inhibits serotonin and/or norepinephrine reuptake by the presynaptic neuronal membrane pump that works to reduce pain. Gabapentin (Neurontin) Inhibiting excitatory neurotransmitter release to reduce neuropathic symptoms Pregabalin (Lyrica) Inhibiting excitatory neurotransmitter release to reduce neuropathic symptoms Carbamazepine (Tegretol) Exerts antineuralgic and muscle relaxant effects Tramadol (Ultram) Binds to μ-opiate receptors in the CNS causing inhibition of ascending pain pathway [Show More]
Last updated: 1 year ago
Preview 1 out of 27 pages
Instant download
Buy this document to get the full access instantly
Instant Download Access after purchase
Add to cartInstant download
Reviews( 0 )
Document information
Connected school, study & course
About the document
Uploaded On
Oct 21, 2022
Number of pages
27
Written in
Additional information
This document has been written for:
Uploaded
Oct 21, 2022
Downloads
0
Views
40













 (1).png)











