Physics > QUESTIONS & ANSWERS > Questions and Answers > 1. The triple point of water occurs at T = 273.16 K. What is this in Fahrenh (All)
Questions and Answers > 1. The triple point of water occurs at T = 273.16 K. What is this in Fahrenheit? 1. Find the temperature at which the Fahrenheit value is exactly twice that of the Celsius value., 1. At what temperature does the Celsius scale equal with the Fahrenheit scale? 1. Which of the following processes requires absorption of heat?
Document Content and Description Below
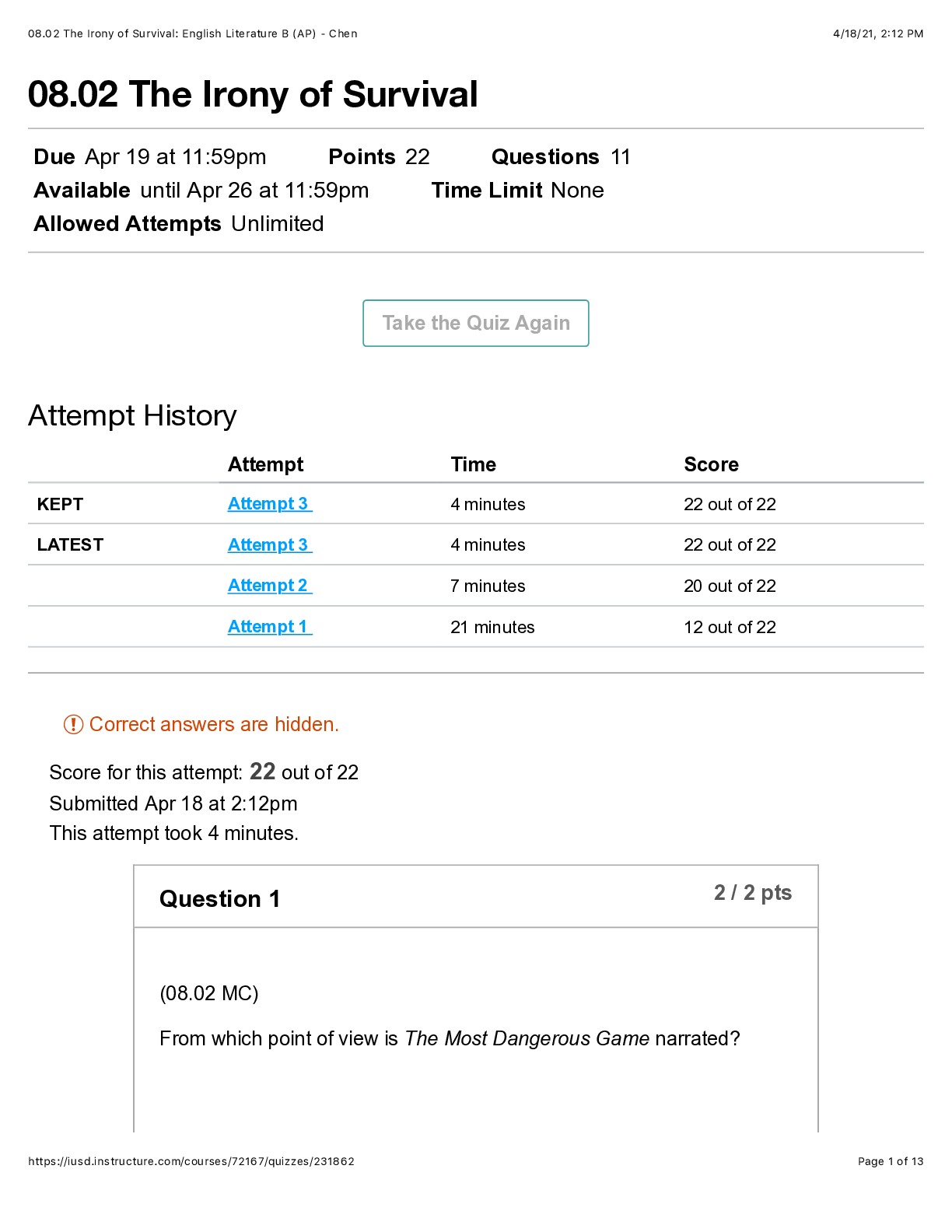
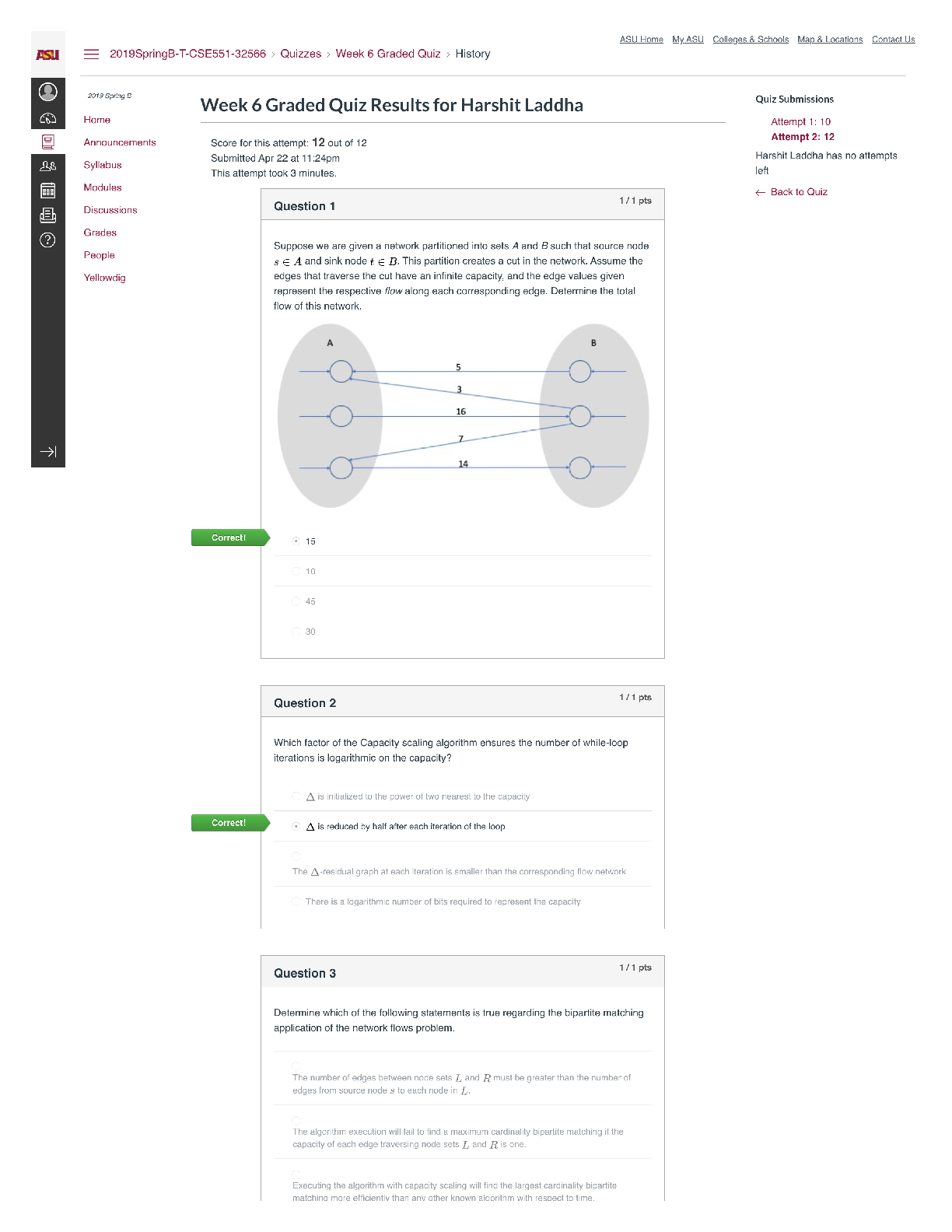
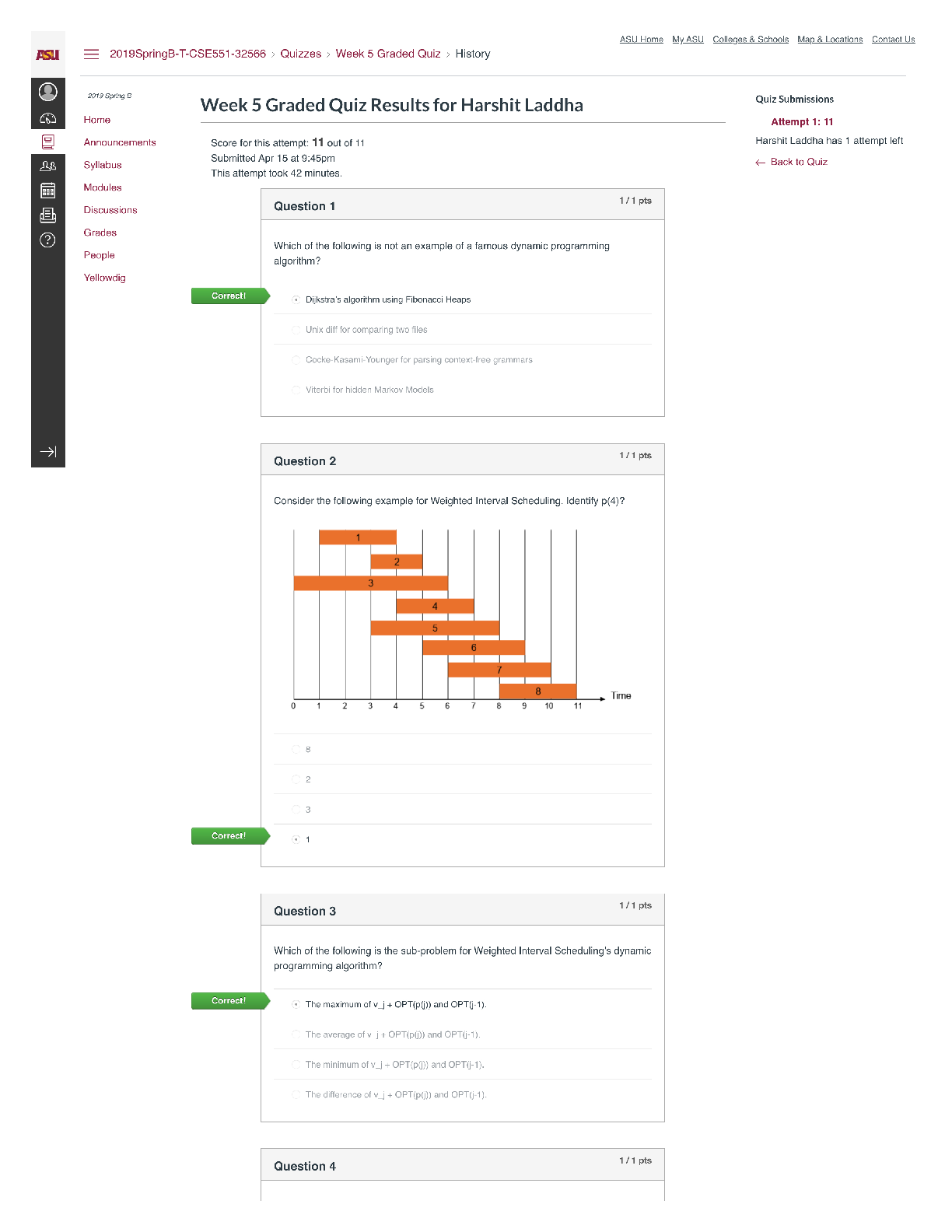
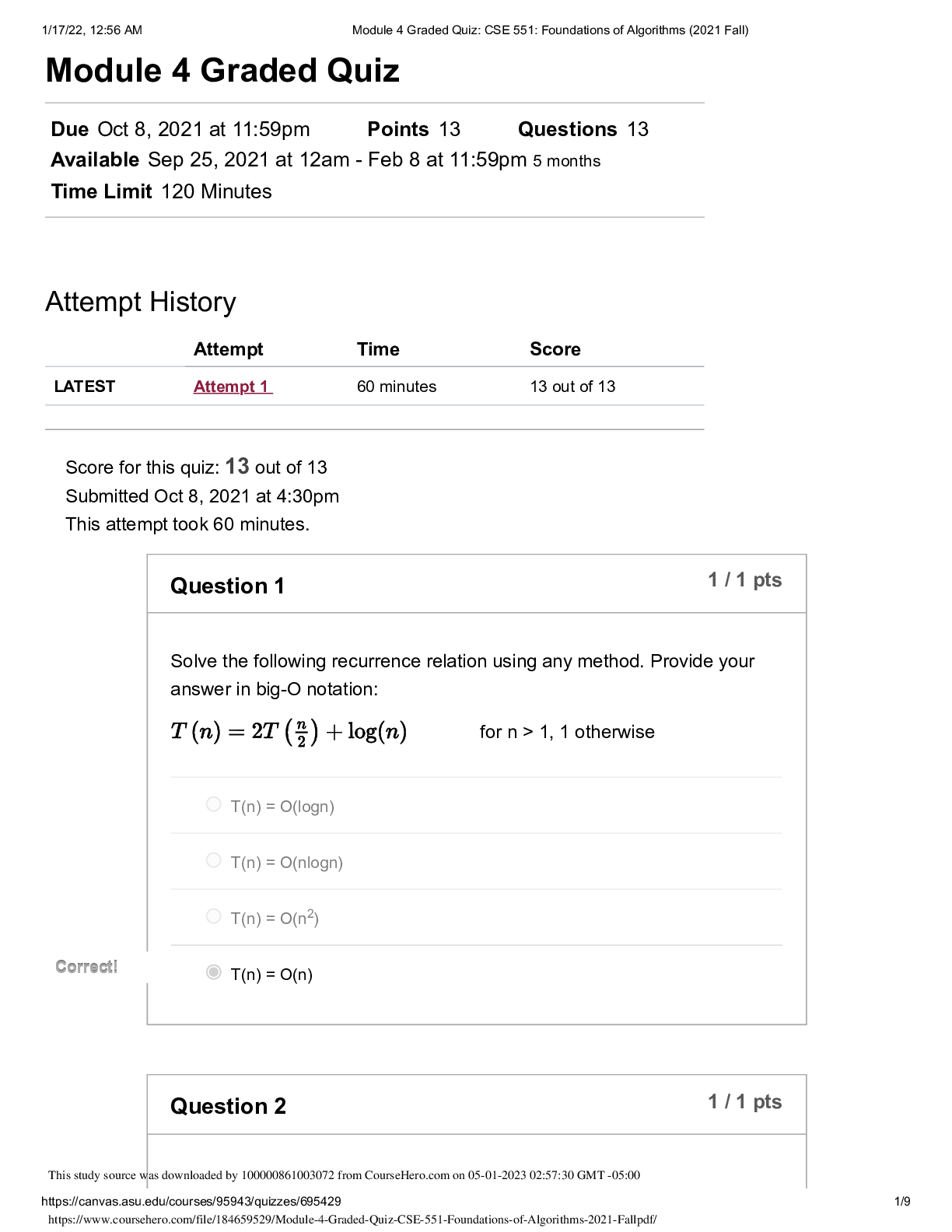
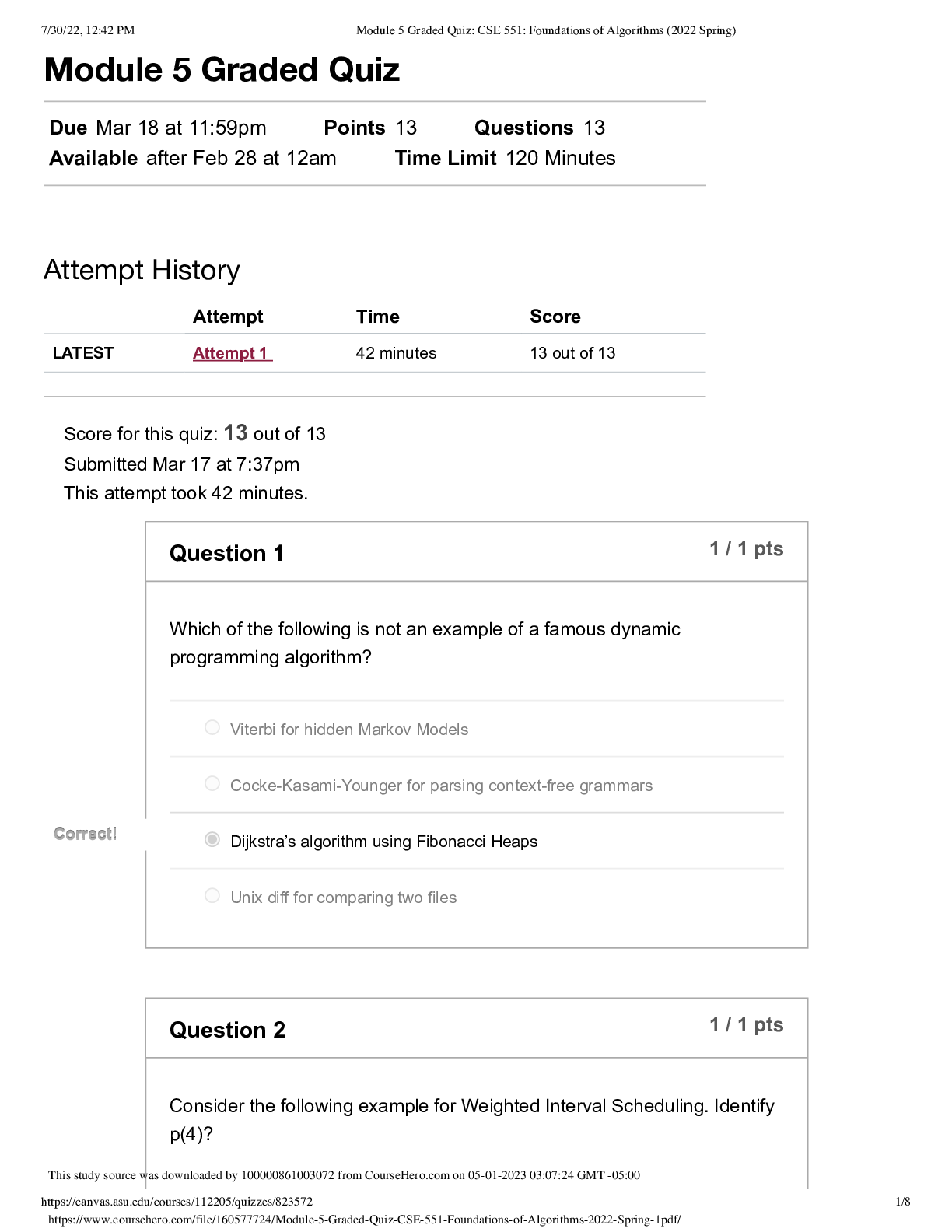
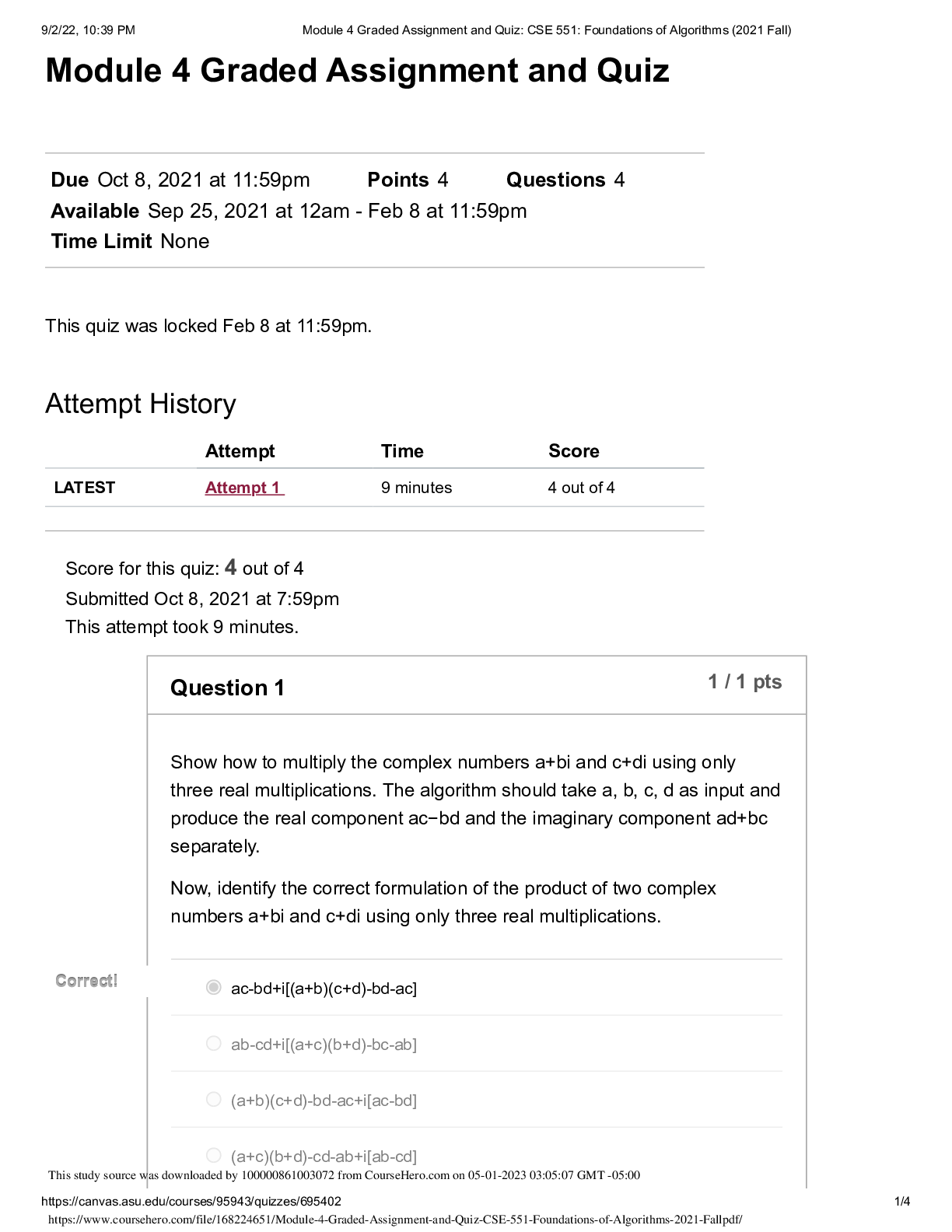
QUESTION 1 1. The triple point of water occurs at T = 273.16 K. What is this in Fahrenheit? A. -17.7720F B. 32.0180F C. 133.980F D.212.020F E. 523.690F 1 points QUESTION 2 1. Find the temper... ature at which the Fahrenheit value is exactly twice that of the Celsius value. A. 00C B. 22.20C C. 35.60C D.80.00C E. 1600C 1 points QUESTION 3 1. At what temperature does the Celsius scale equal with the Fahrenheit scale? A. -8.0 B. 8.0 C. 26.6 D.-40.0 E. 40.0 1 points QUESTION 4 1. Body L is in thermal equilibrium with body U. Body R is also in thermal equilibrium with body U. Which of the following comparisons best describes the temperature of each bodies? Let T be the notation for temperature. A. TL = TU ; TR = TU; TR = 0.5 TL B. TL = TU ; TR = TU; TR = 2 TL C. TL = TU ; TR = TU; TR = 0.25 TL D. TL = TU ; TR = TU; TR = 4 TL E. TL = TU ; TR = TU; TR = TL 1 points QUESTION 5 1. Which of the following processes requires absorption of heat? A. Condensation and Freezing B. Evaporation and Melting C. Condensation and Melting D.Evaporation and Freezing E. All four processes requires heat absorption. 1 points QUESTION 6 1. How much energy is needed to change the temperature of 2.00 kg of liquid water from 30.0ºC to 33.0 ºC? The specific heat of water is 4186 J/kg.0C. A. 50.2 x 103 J B. 92.0 J C. 184 J D.25.1 x 103 J E. 0 J 1 points QUESTION 7 1. Which of the following statements is TRUE as ice changes phase to liquid water? I. Heat is released as ice increases its temperature from a negative value to 00C. II. Heat is released to change the phase of solid ice to liquid water. III. Heat is absorbed by ice as it changes phase to liquid at increasing temperature. A. I only B. II only C. III only D.II and III E. All three statements are false. 1 points QUESTION 8 1. Which of the following processes involves changes in temperature? A. Freezing of liquid water to ice B. Condensation of water vapor to liquid dews C. Evaporation of ocean water D.Sublimation of moth balls E. None of the four processes involve changes in temperature. 1 points QUESTION 9 1. Which of the following processes releases heat? A. Melting of solid ice to liquid water B. Evaporation of liquid water to steam C. Slight increase in your body temperature D.Freezing of a molten magma E. either A and B 1 points QUESTION 10 1. How much energy is needed to change the phase of 2.00 kg of liquid water at 0.0 ºC to ice? The latent heat of fusion of water is 334,000 J/kg. A. 668,000 J B. -668,000 J C. 334,000 J D.0 J E. -334,000 J 1 points QUESTION 11 1. A small electric immersion heater is used to heat 1 kg of water for a cup of instant coffee. The heater is labeled “150 watts” (it converts electrical energy to thermal energy at this rate). Calculate the time required to bring all this water from 250C to 1000C, ignoring any heat losses. A. 176.6 s B. 188.4 s C. 1354 s D.1962 s E. 2093 s 1 points QUESTION 12 1. Materials A, B, and C are solids that are at their melting temperatures. Material A requires 200 J to melt 4 kg, material B requires 300 J to melt 5 kg, and material C requires 400 J to melt 6 kg. Which of the three materials has the highest heat of fusion? A. A B. B C. C D.A and B E. A and C 1 points QUESTION 13 1. Which of the following processes will require the greatest amount of heat? The specific heats of water and steam are 4186 J/(kg•K) and 1860 J/(kg•K), respectively and the latent heats of fusion and vaporization of water are 334,000 J/kg and 2,256,000 J/kg, respectively. A. Raising the temperature of 1.0 kg steam by 100C B.Melting of 1.0 kg ice at 00C C. Raising the temperature of 1.0 kg water by 100C D.Vaporizing 1.0 kg water at 1000C E. All of the above will require the same amount of heat. 1 points QUESTION 14 1. What is the minimum amount of iron needed to melt 1.45 kg of ice initially at -2.35 ˚C if the iron is initially heated to a temperature of 75.0 ˚C? specific heat of iron= 470 J/(kg•K) specific heat of ice (near 0 °C) = 2,100 J/(kg•K) latent of fusion of water= 334,000 J/kg A. 0.203 kg B. 0.404 kg C. 12.7 kg D.13.9 kg E. 17.1 kg 1 points QUESTION 15 1. A 10 kg of ice at 0 °C is mixed with 4 kg of steam at 100 °C in a thermally isolated container and allowed to reach thermal equilibrium. What is the amount of heat needed to melt the ice? The latent heats of fusion and vaporization of water is 334000 J/kg and 2256000 J/kg, respectively and the specific heat of liquid water is 4186 J/kg.K. A. 167,000 J B. 334,000 J C. 3,340,000 J D.2,256,000 J E. 22,560,000 J 1 points QUESTION 16 1. A 10 kg of ice at 0 °C is mixed with 4 kg of steam at 100 °C in a thermally isolated container and allowed to reach thermal equilibrium. What is the amount of heat released to condense the steam into liquid water? The latent heats of fusion and vaporization of water is 334000 J/kg and 2256000 J/kg, respectively and the specific heat of liquid water is 4186 J/kg.K. A. -668,000 J B. 1,336,000 J C. -1,336,000 J D.9,024,000 J E. -9,024,000 J 1 points QUESTION 17 1. A 10 kg of ice at 0 °C is mixed with 4 kg of steam at 100 °C in a thermally isolated container and allowed to reach thermal equilibrium. What is the final temperature of the resulting mixture? Hint: ice melts into water while steam condenses to water before the total mixture reaches thermal equilibrium. The latent heats of fusion and vaporization of water is 334000 J/kg and 2256000 J/kg, respectively and the specific heat of liquid water is 4186 J/kgK. A. 273 K B. 300 K C. 373 K D.399 K E. 689 K 1 points QUESTION 18 1. One way to keep the contents of a garage from becoming too cold on a night when a severe subfreezing temperature is forecast is to put a tub of water in the garage. If the mass of the water is 120 kg and its initial temperature is 250C, how much energy must the water transfer to its surroundings in order to freeze completely? For water, its latent heat of fusion is 330000 J/kg and it freezes at 00C and its specific heat is 4186 J/kg.0C. A. 12.6 x 106 J B. 27.4 x 106 J C. 40.0 x 106 J D. 52.1 x 106 J E. 60.0 x 106 J 1 points QUESTION 19 1. Most materials expands when heated except ______. A. Aluminum B. Brass C. Iron D.Steel E. Water 1 points QUESTION 20 1. The ratio of the change in length with the change in temperature is equal to _____. Let α be the coefficient of linear expansion and Lo is the original length. A. α B. Lo C. αLo D. α/Lo E. Lo/α 1 points QUESTION 21 1. The ratio of the change in volume of a solid to its initial volume gives _____. A. the coefficient of volume expansion B. the coefficient of linear expansion C. the change in temperature D.the product of the coefficient of linear expansion and the change in temperature E. the product of the coefficient of volume expansion and the change in temperature 1 points QUESTION 22 1. A steel ruler of has mass 50.000 g and length 30.000 cm at 27.000oC. What is its length at 77oC? Coefficient of linear expansion of steel is α = 1.2000 x 10-5/K. A. 29.964 cm B. 29.982 cm C. 30.000 cm D.30.018 cm E. 30.036 cm 1 points QUESTION 23 1. Jonard is carrying a steel meterstick (with 1.0000 m) while waiting along the corridors (with temperature 35 °C). He then enters the physics lab where the stick shrinks to 0.9997 m. What is the temperature inside the lab? Note that for steel: α = 12 x 10-6/0C A. 100C B. 200C C. 300C D. 260C E. 140C 1 points QUESTION 24 1. A steel ruler of has mass 50.000 g and length 30.000 cm at 27.000oC. At what temperature will its length becomes 30.026 cm? Coefficient of linear expansion of steel is α = 1.2000 x 10-5 /K. A. 00C B. 270C C. 730C D.1000C E. 1270C 1 points QUESTION 25 1. A metal rod is 200.00 cm long at 00C and 200.28 cm long at 600C. What is its coefficient of linear expansion? A. 11 x 10-6/0C B. 17 x 10-6/0C C. 23 x 10-6/0C D. 28 x 10-6/0C E. 51 x 10-6/0C 1 points QUESTION 26 1. What temperature change would require a 0.101% increase in length of a copper wire with initial length 1.25 m? Coefficient of linear expansion of copper, a = 1.70 x10-5 /K. A. 32.5 K B. 47.5 K C. 59.4 K D.215 K E. 752 K 1 points QUESTION 27 1. A round hole of 6.000 cm in diameter at 00C is cut in a sheet of brass(alpha = 0.000018 /0C). Find the new diameter of the hole at 500C. A. 3.002 cm B. 6.004 cm C. 0.302 cm D.0.604 cm E. 0.032 cm 1 points QUESTION 28 1. The coefficient of linear expansion of steel is α=11 × 10-6/C°. A steel ball has a volume of exactly 100 cm3 at 0º C. When heated to 100° C, its volume increases by: A. 1.23 cm3 B. 0.33 cm3 C. 0.0033 cm3 D.0.0011 cm3 E. 0.000011 cm3 1 points QUESTION 29 1. A glass bottle (βglass = 2.6 x 10-5 /0C) is half-filled with oil (βoil = 70 x 10- 5 /0C). Suppose that your eyes are able to detect very minute changes in dimensions. If the temperature is increased by 1000C, what would you observe? A.The glass bottle’s volume would decrease. B.The glass bottle’s volume would not change. C. The glass bottle’s volume would appear half empty. D.The oil’s level would increase. E. The oil’s level would decrease. 1 points QUESTION 30 1. A steel ball with a radius of 3.50 cm undergoes expansion after its temperature increased by 27.3 K. What is the change in its volume? Coefficient of linear expansion of steel, a = 1.20 x 10-5/K. A. 2.54 cm3 B. 3.56 cm3 C. 4.68 cm3 D.0.177 cm3 E. 0.00588 cm3 [Show More]
Last updated: 1 year ago
Preview 1 out of 8 pages

Buy this document to get the full access instantly
Instant Download Access after purchase
Add to cartInstant download
We Accept:

Reviews( 0 )
$4.00
Document information
Connected school, study & course
About the document
Uploaded On
Jan 17, 2023
Number of pages
8
Written in
Additional information
This document has been written for:
Uploaded
Jan 17, 2023
Downloads
0
Views
56







.png)

 answers.png)


 REVISION 2021 Graded A, Latest Questions and Answers with Explanations, All Correct Study Guide, Download to Score A.png)
 REVISION 2021 Graded A, Latest Questions and Answers with Explanations, All Correct Study Guide, Download to Score A.png)
 & ACUTE, ABDOMEN) REVISION 2021 Graded A, Latest Questions and Answers with Explanations, All Correct Study Guide, Download to Score A.png)

 70 Test Questions with Answers Graded A, Latest Questions and Answers with Explanations, All Correct Study Guide, Download to Score A.png)


