Chemistry > EXAM > Chem 103 Module 1 to 6 Exam with Verified answers (100 OUT OF 100) Portage learning (Latest Update) (All)
Chem 103 Module 1 to 6 Exam with Verified answers (100 OUT OF 100) Portage learning (Latest Update)
Document Content and Description Below
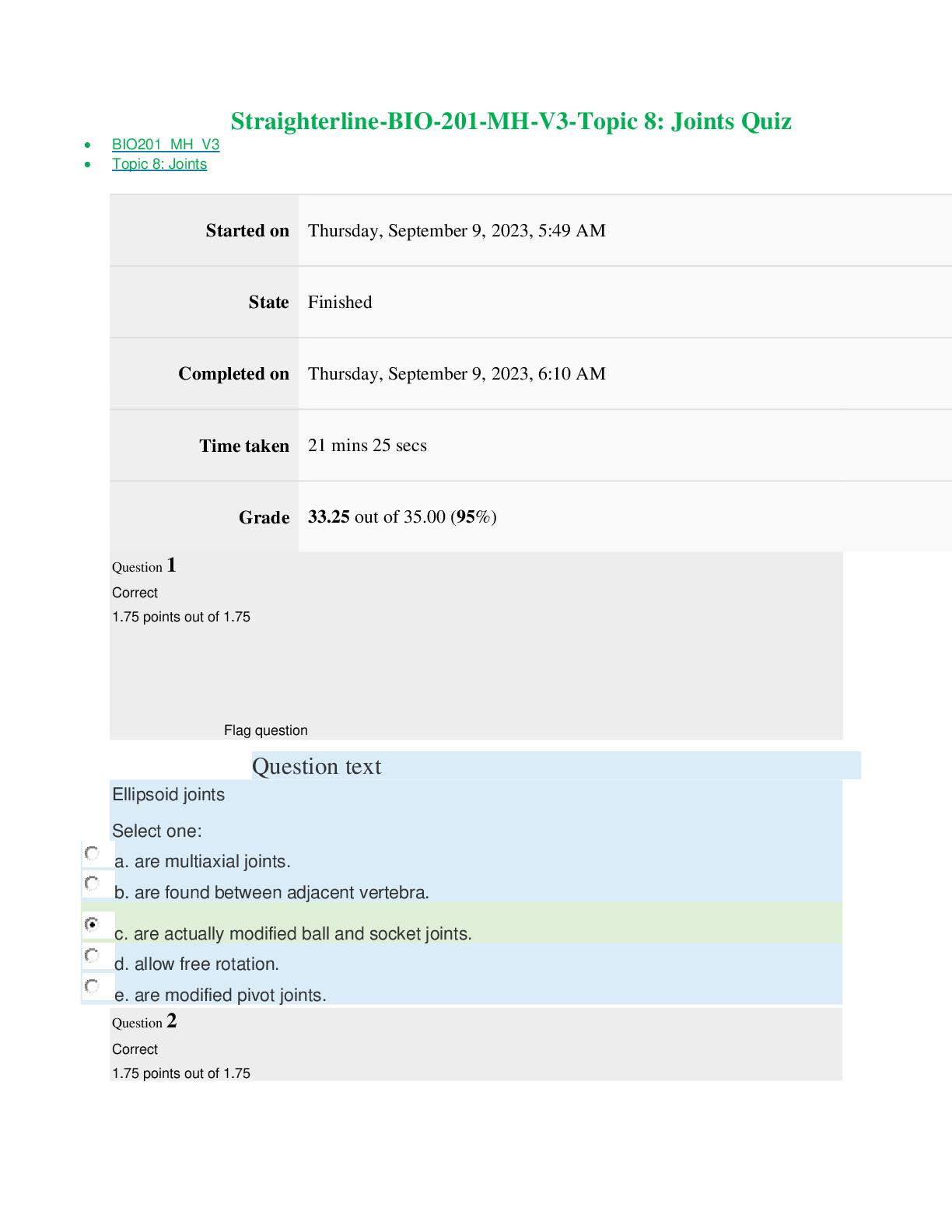
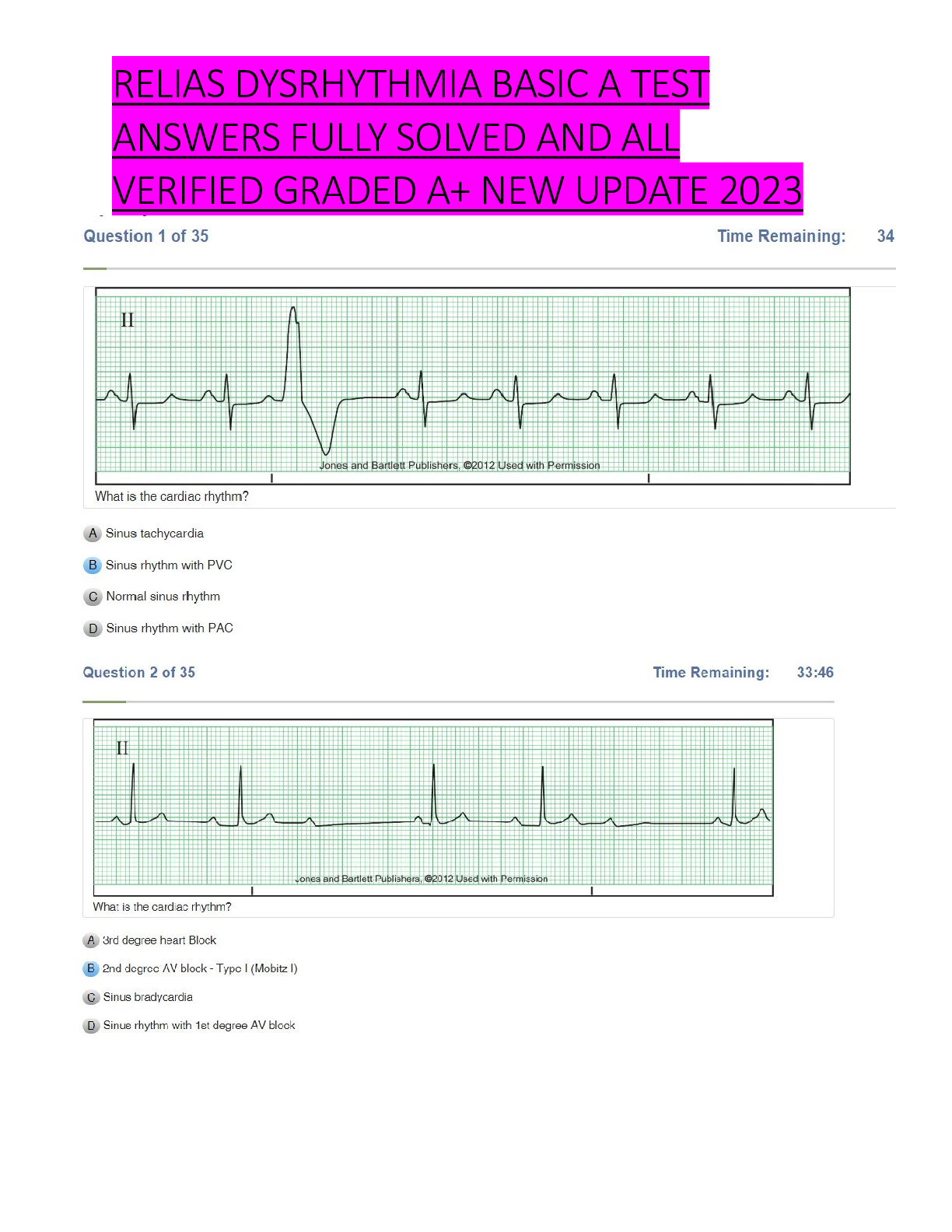
Chem 103 Module 1 to 6 Exam with Verified answers (100 OUT OF 100) Portage learning (Latest Update) MODULE 1 EXAM Question 1 Click this link to access the Periodic Table. This may be helpful throughou... t the exam. 1. Convert 845.3 to exponential form and explain your answer. 2. Convert 3.21 x 10-5 to ordinary form and explain your answer. 1. Convert 845.3 = larger than 1 = positive exponent, move decimal 2 places = 8.453 x 102 2. Convert 3.21 x 10-5 = negative exponent = smaller than 1, move decimal 5 places = 0.0000321 Question 2 Click this link to access the Periodic Table. This may be helpful throughout the exam. Using the following information, do the conversions shown below, showing all work: 1 ft = 12 inches 1 pound = 16 oz 1 gallon = 4 quarts 1 mile = 5280 feet 1 ton = 2000 pounds 1 quart = 2 pints kilo (= 1000) 1/100) milli (= 1/1000) deci (= 1/10) centi (= 1. 24.6 grams = ? kg 2. 6.3 ft = ? inches 1. 24.6 grams x 1 kg / 1000 g = 0.0246 kg 2. 6.3 ft x 12 in / 1 ft = 75.6 inches please always use the correct units in your final answer Question 3 Click this link to access the Periodic Table. This may be helpful throughout the exam. Do the conversions shown below, showing all work: 1. 28oC = ? oK 2. 158oF = ? oC 3. 343oK = ? oF 1. 28oC + 273 = 301 oK oC → oK (make larger) +273 2. 158oF - 32 ÷ 1.8 = 70 oC oF → oC (make smaller) -32 ÷1.8 3. 343oK - 273 = 70 oC x 1.8 + 32 = 158 oF oK → oC → oF Question 4 Click this link to access the Periodic Table. This may be helpful throughout the exam. Be sure to show the correct number of significant figures in each calculation. 1. Show the calculation of the mass of a 18.6 ml sample of freon with density of 1.49 g/ml 2. Show the calculation of the density of crude oil if 26.3 g occupies 30.5 ml. 1. M = D x V = 1.49 x 18.6 = 27.7 g 2. D = M / V = 26.3 / 30.5 = 0.862 g/ml Question 5 Click this link to access the Periodic Table. This may be helpful throughout the exam. 1. 3.0600 contains ? significant figures. 2. 0.0151 contains ? significant figures. 3. 3.0600 ÷ 0.0151 = ? (give answer to correct number of significant figures) 1. 3.0600 contains 5 significant figures. 2. 0.0151 contains 3 significant figures. 3. 3.0600 ÷ 0.0151 = 202.649 = 203 (to 3 significant figures for 0.0151) Question 6 Click this link to access the Periodic Table. This may be helpful throughout the exam. Classify each of the following as an element, compound, solution or heterogeneous mixture and explain your answer. 1. Coca cola 2. Calcium 3. Chili 1. Coca cola - is not on periodic table (not element) - no element names (not compound) appears to be one substance = Solution 2. Calcium - is on periodic table = Element 3. Chili - is not on periodic table (not element) - no element names (not compound) appears as more than one substance (meat, beans, sauce) = Hetero Mix Question 7 Click this link to access the Periodic Table. This may be helpful throughout the exam. Classify each of the following as a chemical change or a physical change 1. Charcoal burns 2. Mixing cake batter with water 3. Baking the batter to a cake 1. Charcoal burns - burning always = chemical change 2. Mixing cake batter with water - mixing = physical change 3. Baking the batter to a cake - baking converts batter to new material = chemical change Click this link to access the Periodic Table. This may be helpful throughout the exam. Show the full Nuclear symbol including any + or - charge (n), the atomic number (y), the mass number (x) and the correct element symbol (Z) for each element for which the protons, neutrons and electrons are shown - symbol should appear as follows: xZy+/- n 31 protons, 39 neutrons, 28 electrons 31 protons = Ga31, 39 neutrons = 70Ga31, 28 electrons = (+31 - 28 = +3) = 70Ga +3 Question 9 Click this link to access the Periodic Table. This may be helpful throughout the exam. Name each of the following chemical compounds. Be sure to name all acids as acids (NOT for instance as binary compounds) 1. PF5 2. Al2(CO3)3 3. H2CrO4 1. PF5 - binary molecular = phosphorus pentafluoride 2. Al2(CO3)3 - nonbinary ionic = aluminum carbonate 3. H2CrO4 - nonbinary acid = chromic acid incorrect fluoride prefix Question 10 Click this link to access the Periodic Table. This may be helpful throughout the exam. Write the formula for each of the following chemical compounds explaining the answer with appropriate charges and/or prefixes and/or suffixes. 1. Carbon monoxide 2. Manganese (IV) acetate 3. Phosphorous acid 1. Carbon monoxide - ide = binary, mono = 1 O = CO 2. Manganese (IV) acetate - Mn+4, C2H3O -1 = Mn(C2H3O2)4 3. Phosphorous acid - nonbinary acid of H + phosphite (PO -3) = H PO MODULE 2 EXAM Question 1 Click this link to access the Periodic Table. This may be helpful throughout the exam. Show the calculation of the molecular weight for the following compounds, reporting your answer to 2 places after the decimal. 1. Al2(CO3)3 2. C8H6NO4Cl 1. 2Al + 3C + 9O = 233.99 2. 8C + 6H + N + 4O + Cl = 215.59 Question 2 Click this link to access the Periodic Table. This may be helpful throughout the exam. Show the calculation of the number of moles in the given amount of the following substances. Report your answerto 3 significant figures. 1. 13.0 grams of (NH4)2CO3 2. 16.0 grams of C8H6NO4Br 1. Moles = grams / molecular weight = 13.0 / 96.09 = 0.135 mole 2. Moles = grams / molecular weight = 16.0 / 260.04 = 0.0615 mole Question 3 Click this link to access the Periodic Table. This may be helpful throughout the exam. Show the calculation of the number of grams in the given amount of the following substances. Report your answer to 1 place after the decimal. 1. 1.20 moles of (NH4)2CO3 2. 1.04 moles of C8H6NO4Br 1. Grams = Moles x molecular weight = 1.20 x 96.09 = 115.3 grams 2. Grams = Moles x molecular weight = 1.04 x 260.04 = 270.4 grams Question 4 Click this link to access the Periodic Table. This may be helpful throughout the exam. Show the calculation of the percent of each element present in the following compounds. Report your answer to 2 places after the decimal. 1. (NH4)2CrO4 2. C8H8NOI 1. %N = 2 x 14.01/152.08 x 100 = 18.43% %H = 8 x 1.008/152.08 x 100 = 5.30% %Cr = 1 x 52.00/152.08 x 100 = 34.20% %O = 4 x 16.00/152.08 x 100 = 42.08% 2. %C = 8 x 12.01/261.05 x 100 = 36.80% %H = 8 x 1.008/261.05 x 100 = 3.09% %N = 1 x 14.01/261.05 x 100 = 5.37% %O = 1 x 16.00/261.05 x 100 = 6.13% %I = 1 x 126.9/261.05 x 100 = 48.61% Question 5 Click this link to access the Periodic Table. This may be helpful throughout the exam. Show the calculation of the empirical formula for each compound whose elemental composition is shown below. 38.76% Ca, 19.87% P, 41.27% O 38.76% Ca / 40.08 = 0.9671 / 0.6416 = 1.5 x 2 = 3 19.87% P / 30.97 = 0.6416 / 0.6416 = 1 x 2 = 2 41.27% O / 16.00 = 2.579 / 0.6416 = 4 x 2 = 8 → Ca3P2O8 Question 6 Click this link to access the Periodic Table. This may be helpful throughout the exam. Balance each of the following equations by placing coefficients in front of each substance. 1. C6H6 + O2 → CO2 + H2O 2. As + O2 → As2O5 3. Al2(SO4)3 + Ca(OH)2 → Al(OH)3 + CaSO4 1. 2 C6H6 + 15 O2 → 12 CO2 + 6 H2O 2. 4 As + 5 O2 → 2 As2O5 3. Al2(SO4)3 + 3 Ca(OH)2 → 2 Al(OH)3 + 3 CaSO4 Question 7 Click this link to access the Periodic Table. This may be helpful throughout the exam. Classify each of the following reactions as either: Combination Decomposition Combustion Double Replacement Single Replacement 1. H2SO4 → SO3 + H2O 2. S + 3 F2 → SF6 3. H2 + NiO → Ni + H2O 1. H2SO4 → SO3 + H2O = Decomposition, One reactant → Two Products 2. S + 3 F2 → SF6 = Combination. Two reactants→ One product 3. H2 + NiO → Ni + H2O = Single Replacement, Hydrogen displaces metal ion Question 8 Click this link to access the Periodic Table. This may be helpful throughout the exam. Show the calculation of the oxidation number (charge) of ONLY the atoms which are changing in the following redox equations. Na2HAsO3 + KBrO3 + HCl → NaCl + KBr + H3AsO4 Na2HAsO3 + KBrO3 + HCl → NaCl + KBr + H3AsO4 Na2HAsO3: Na is metal in group I = +1 (total is +2), H = +1, each O is -2 (total is -6), so As is +3 H3AsO4: H is +1 (total is +3), each O is -2 (total is -8), so As is +5 KBrO3: K is metal in group I = +1, each O is -2 (total is -6), so Br is +5 KBr: K is metal in group I = +1, so Br is -1 Question 9 Click this link to access the Periodic Table. This may be helpful throughout the exam. Show the balancing of the following redox equation, including the determination of the oxidation number (charge) of ONLY the atoms which are changing. KMnO4 + KI + H2O → KIO3 + MnO2 + KOH Mn compounds x 2 ; I compounds x 1 = 2KMnO4 + 1 KI + 1 H2O → 1 KIO3 + 2MnO2 + 2KOH KMnO4 + KI + H2O → KIO3 + MnO2 + KOH KMnO4: K is metal in group I = +1, each O is -2 (total is -8), so Mn is +7 MnO2: Each O is -2 (total is -4), so Mn is +4 KI: K is metal in group I = +1, so I is -1 KIO3: K is metal in group I = +1, each O is -2 (total is -6), so I is +5 Since Mn (on left side) is +7 and Mn (on right side) is +4: Mn changes by 3 Since I (on left side) is -1 and I (on right side) is +5: I changes by 6 Multiply Mn compounds by 2 and I compounds by 1 and after balancing other atoms = 2 KMnO4 + 1 KI + 1 H2O → 1 KIO3 + 2 MnO2 + 2 KOH Question 10 Click this link to access the Periodic Table. This may be helpful throughout the exam. Show the balanced equation and the calculation of the number of moles and grams of CO2 formed from 20.6 grams of C6H6. Show your answers to 3 significant figures. C6H6 + O2 → CO2 + H2O Molar mass of CO2 = 44.01 g/mol mass of CO2 = 1.5822 ml x 44.01 g/mol =69.63 grams mass of CO2 = 69.63 grams moles of CO2 = 1.58 mole 2 C6H6 + 15 O2 → 12 CO2 + 6 H2O 20.6 g / (6 x 12.01 + 6 x 1.008) = 20.6 / 78.108 = 0.2637 mole x 12/2 = 1.58 mole CO2 1.582 mole CO2 x (12.01 + 2 x 16.00) = 69.6 g CO2 MODULE 3 EXAM Click this link to access the Periodic Table. This may be helpful throughout the exam. A reaction between HCl and NaOH is being studied in a styrofoam coffee cup with NO lid and the heat given off is measured by means of a thermometer immersed in the reaction mixture. Enter the correct thermochemistry term to describe the item listed. 1. The type of thermochemical process 2. The amount of heat released in the reaction of HCl with NaOH 1. Heat given off = Exothermic process 2. The amount of heat released = Heat of reaction Question 2 Click this link to access the Periodic Table. This may be helpful throughout the exam. 1. Show the calculation of the final temperature of the mixture when a 22.8 gram sample of water at 74.6oC is added to a 14.3 gram sample of water at 24.3oC in a coffee cup calorimeter. c (water) = 4.184 J/g oC 2. Show the calculation of the energy involved in freezing 54.3 grams of water at 0oC if the Heat of Fusion for water is 0.334 kJ/g 1. - (mwarn H2O x cwarn H2O x ∆twarn H2O) = (mcool H2O x ccool H2O x ∆tcool H2O) - [22.8 g x 4.184 J/g oC x (Tmix - 74.6oC)] = [14.3 g x 4.184 J/g oC x (Tmix - 24.3oC)] - [95.3952 J/oC x (Tmix - 74.6oC)] = [59.8312 J/oC x (Tmix - 24.3oC)] Tmix = 55.2oC 2. ql↔s = m x ∆Hfusion = 54.3 g x 0.334 kJ/g = 18.14 kJ (since heat is removed) = - 18.14 kJ Question 3 Click this link to access the Periodic Table. This may be helpful throughout the exam. Show the calculation of the amount of heat involved if 35.6 g of H2S is reacted with excess O2 to yield sulfur trioxide and water by the following reaction equation. Report your answer to 4 significant figures. 2 H2S (g) + 3 O2 (g) → 2 SO2 (g) + 2 H2O (g) ΔH = - 1124 kJ 1 mol H2s = 34.1 g of H2S = 603.2 kj (35.6g/34.1 g) x -603.2 kJ = (1.0439) x (-603.2 kJ) = -629. 7 kj 2 H2S (g) + 3 O2 (g) → 2 SO2 (g) + 2 H2O (g) ΔH = - 1124 kJ ΔHrx is for 2 mole of H2S reaction uses 35.6 g of H2S = 35.6/34.086 = 1.044 mole of H2S q = ΔHrx x new moles / original moles q = -1124 kJ x 1.044 mole of H2S / 2 mole H2S = 586.7 given off Question 4 Click this link to access the Periodic Table. This may be helpful throughout the exam. Show the calculation of the heat of reaction (ΔHrxn) for the reaction: 3 C (graphite) + 4 H2 (g) → C3H8 (g) by using the following thermochemical data: C (graphite) kJ + O2 (g) → CO2 (g) ΔH = - 393.51 2 H2 (s) kJ + O2 (g) → 2 H2O(l) ΔH = - 571.66 C3H8 (g) + 5 O2 (g) → 3 CO2 (g) + 4 H2O(l) ΔH = - 2220.0 kJ Your Answer: 3 (C (graphite) + O2 (g) → CO2 (g) ΔH = - 393.51 kJ) 2 (2 H2 (s) + O2 (g) → 2 H2O(l) ΔH = - 571.66 kJ) 3 CO2 (g) + 4 H2O(l) → C3H8 (g) + 5 O2 (g) ΔH = + 2220.0 kJ 3 C (graphite) + 4 H2 (g) → C3H8 (g) ΔHrxn = - 103.85 kJ ΔHrxn = 3(- 393.51) + 2(- 571.66) + 2220.0 = - 103.85 Question 5 Click this link to access the Periodic Table. This may be helpful throughout the exam. Show the calculation of the heat of reaction (ΔHrxn) for the reaction: 2 CH4 (g) + 3 O2 (g) → 2 CO (g) + 4 H2O (l) by using the following ΔHf0 data: ΔH 0 CH (g) = -74.6 kJ/mole, ΔH 0 CO (g) = -110.5 kJ/mole, ΔH 0 H O (l) = -285.8 kJ/mole 2 CH4 (g) + 3 O2 (g) → 2 CO (g) + 4 H2O (l) ΔHf0 CH4 (g) = -74.6 kJ/mole, ΔHf0 CO (g) = -110.5 kJ/mole, ΔHf0 H2O (l) = -285.8 kJ/mole ΔHrxn = 2(+74.6) + 3(0) + 2(-110.5) + 4(-285.8) = - 1215.0 kJ/mole Question 6 Click this link to access the Periodic Table. This may be helpful throughout the exam. Show the calculation of the new temperature of a gas sample has an original volume of 740 ml when collected at 710 mm and 35oC when the volume becomes 460 ml at 1.20 atm. P1V1/T1 = P2V2/T2 T2 = 1.2 x 460x308 / 0.934 x 740 = 245.98 kelvin = -27.17oC (Pi x Vi ) / Ti = (Pf x Vf ) / Tf 740 ml/1000 = 0.740 liters = Vi 710 mm/760 = 0.934 atm = Pi 460 ml/1000 = 0.460 liters = Vf 1.20 atm = Pf 35oC + 273 = 308oK = Ti (0.934) x (0.740) / 308 = (1.20) x (0.460) / Tf Tf = 246 oK Question 7 Click this link to access the Periodic Table. This may be helpful throughout the exam. Show the calculation of the volume occupied by a gas sample containing 0.632 mole collected at 710 mm and 35oC. P x V = n x R x T 0.632 mole = n R = 0.0821 710 mm/760 = 0.934 atm = P 35oC + 273 = 308oK = T (0.934) x V = (0.632) x (0.0821) x (308) V = 17.1 liters Question 8 Click this link to access the Periodic Table. This may be helpful throughout the exam. Show the calculation of the volume of CO2 gas formed by the combustion of 25.5 grams of C6H6 at 20oC and 1.25 atm. The combustion of benzene (C6H6) takes place by the following reaction equation. 2 C6H6 (g) + 15 O2 (g) → 12 CO2 (g) + 6 H2O (g) (MW = 78) (MW = 32) (MW = 44) (MW = 18) 2 C6H6 (g) + 15 O2 (g) → 12 CO2 (g) + 6 H2O (g) 25.5 grams 37.75 liters ↓ ↑ by V = nRT / P = (1.9614)(0.0821)(293)/1.25 0.3269 mol → 12/2 x 0.3269 mol Click this link to access the Periodic Table. This may be helpful throughout the exam. Show the calculation of the mole fraction of each gas in a 1.00 liter container holding a mixture of 7.60 g of N2 and 8.40 g of O2 at 25oC. nN2 = gN2 / (MWN2) = 7.60 g / 28.02 = 0.2712 mol nO2 = gO2 / (MWO2) = 8.40 g / 32.00 = 0.2625 mol XN2 = 0.2712 / (0.2712 + 0.2625) = 0.5082 XO2 = 0.2625 / (0.2712 + 0.2625) = 0.4918 Click this link to access the Periodic Table. This may be helpful throughout the exam. Show the calculation of the molecular weight of an unknown gas if the rate of effusion of carbon dioxide gas (CO2) is 1.83 times faster than that of an unknown gas. (rN2 /runknown)2 = MWunknown / MWCO2 (1.83/1)2 = MWunknown / 44.01 MWunknown = (1.83)2 x 44.01 = 147.4 calculation error; minus 2.5 MODULE 4 EXAM Click this link to access the Periodic Table. This may be helpful throughout the exam. Write the subshell electron configuration (i.e.1s2 2s2, etc.) for the Fe26 atom. Fe26 = 26 electrons = 1s2 2s2 2p6 3s2 3p6 4s2 3d6 Click this link to access the Periodic Table.This may be helpful throughout the exam. Write the subshell electron configuration (i.e.1s2 2s2, etc.) for the S16 atom. S16 = 16 electrons = 1s2 2s2 2p6 3s2 3p4 Click this link to access the Periodic Table.This may be helpful throughout the exam. Write the subshell electron configuration (i.e.1s2 2s2, etc.) for the P15 atom and identify which are valence (outer shell) electrons and determine how many valence electrons there are. P15 = 15 electrons = 1s2 2s2 2p6 3s2 3p3 = 5 valence electrons Question 4 Click this link to access the Periodic Table.This may be helpful throughout the exam. * For the following question, use the [Show More]
Last updated: 4 hours ago
Preview 5 out of 35 pages
Loading document previews ...
Buy this document to get the full access instantly
Instant Download Access after purchase
Add to cartInstant download
We Accept:

Reviews( 0 )
$19.00
Document information
Connected school, study & course
About the document
Uploaded On
Jul 19, 2024
Number of pages
35
Written in
Additional information
This document has been written for:
Uploaded
Jul 19, 2024
Downloads
0
Views
5