STUDENT EXPLORATION: EQUILIBRIUM AND CONCENTRATION 2020
Document Content and Description Below
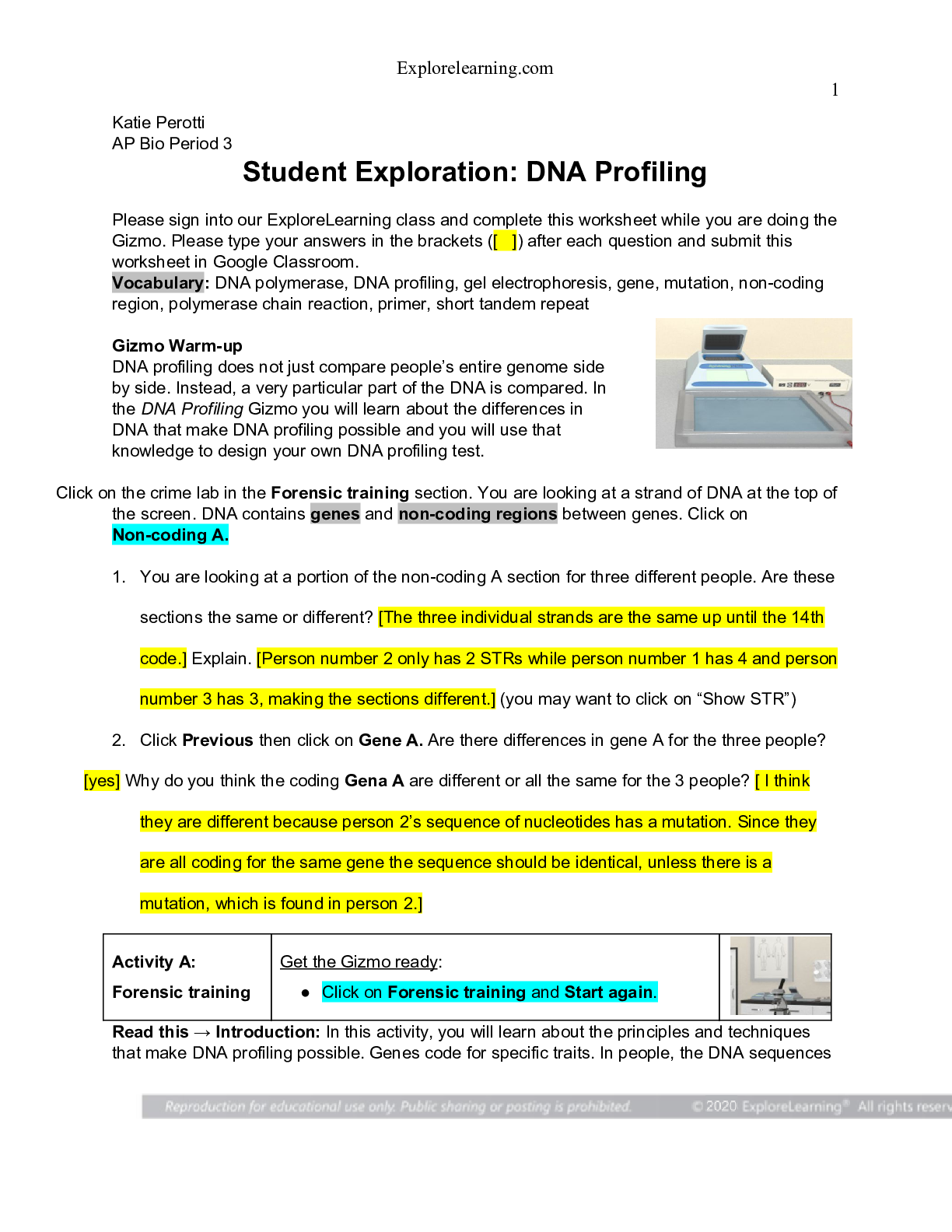
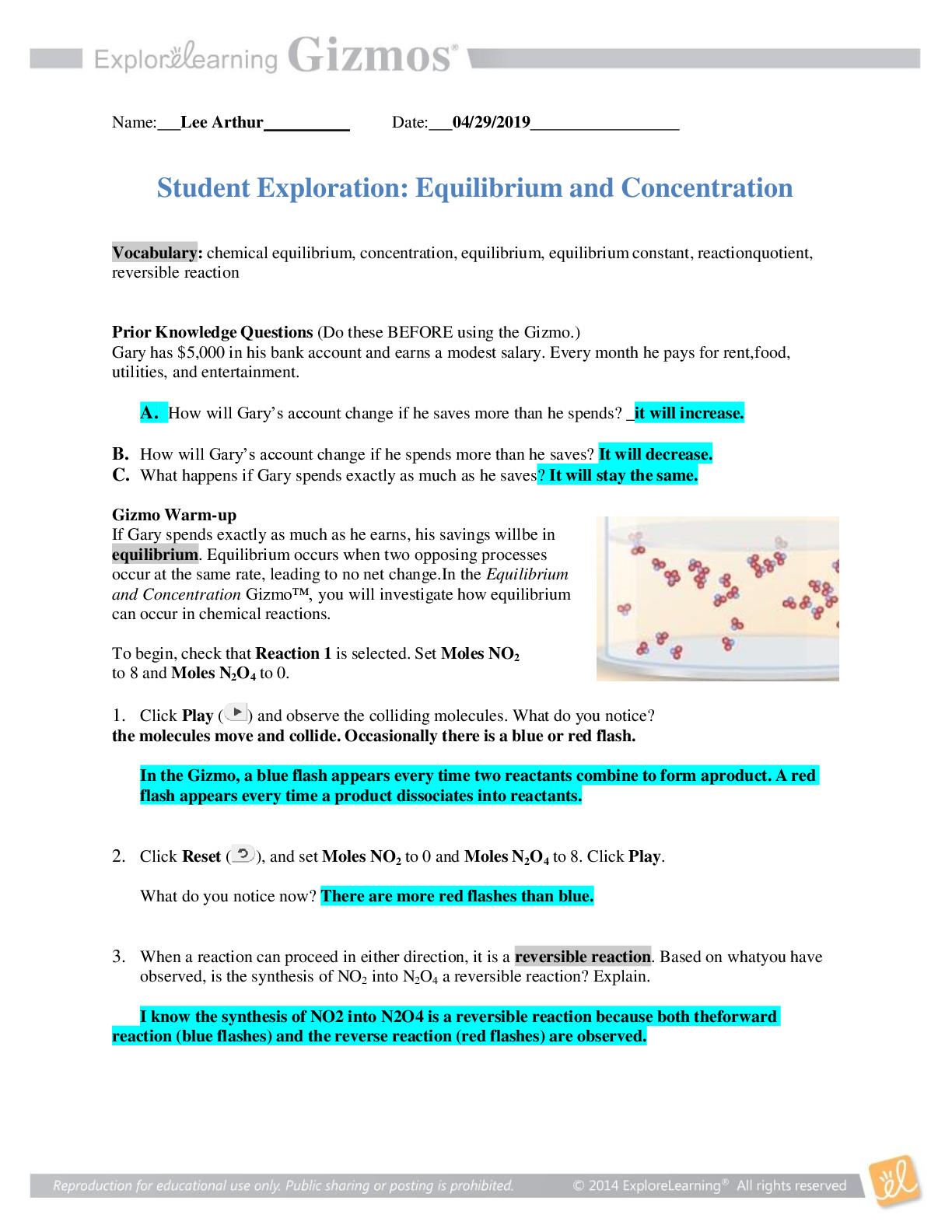
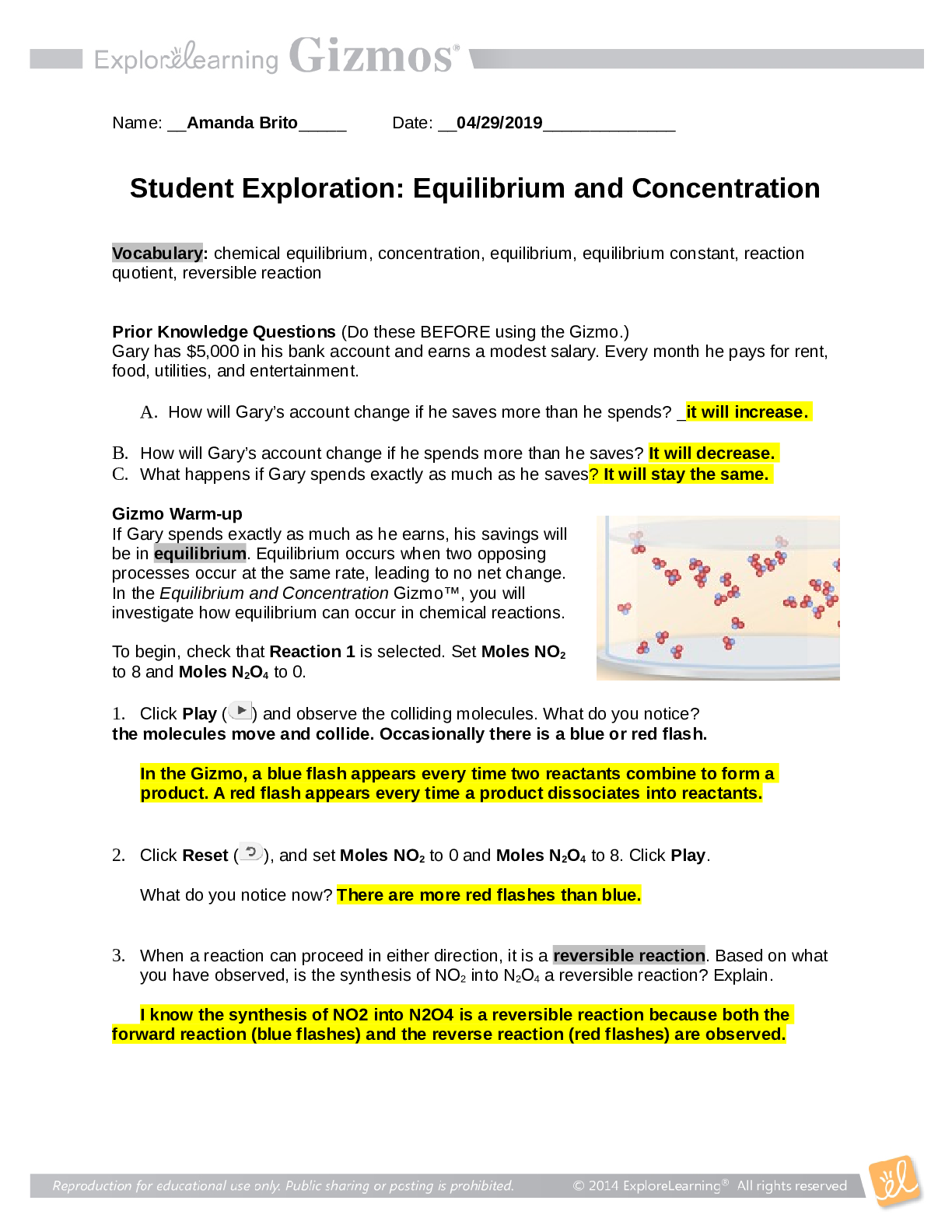
Vocabulary: chemical equilibrium, concentration, equilibrium, equilibrium constant, reaction quotient, reversible reaction Prior Knowledge Questions (Do these BEFORE using the Gizmo.) Gary has $... 5,000 in his bank account and earns a modest salary. Every month he pays for rent, food, utilities, and entertainment. A. How will Gary’s account change if he saves more than he spends? He will have more money B. How will Gary’s account change if he spends more than he saves? The money in his account will decrease C. What happens if Gary spends exactly as much as he saves? The money in his account will remain the same. Gizmo Warm-up If Gary spends exactly as much as he earns, his savings will be in equilibrium. Equilibrium occurs when two opposing processes occur at the same rate, leading to no net change. In the Equilibrium and Concentration Gizmo, you will investigate how equilibrium can occur in chemical reactions. To begin, check that Reaction 1 is selected. Set Moles NO2 to 8 and Moles N2O4 to 0. 1. Click Play ( ) and observe the colliding molecules. What do you notice? The molecules sometimes combine with another to form N2O4. In the Gizmo, a blue flash appears every time two reactants combine to form a product. A red flash appears every time a product dissociates into reactants. 2. Click Reset ( ), and set Moles NO2 to 0 and Moles N2O4 to 8. Click Play. What do you notice now? The N2O4 molecules are breaking apart. 3. When a reaction can proceed in either direction, it is a reversible reaction. Based on what you have observed, is the synthesis of NO2 into N2O4 a reversible reaction? Explain. It is a reversible reaction because NO2 can combine with another NO2 to form N2O4 and N2O4 can break apart to form NO2. Question: What are the characteristics of reversible reactions? 1. Predict: Suppose you began with 8 moles of NO2 in the chamber. What do you think will happen if you let the reaction go for a long time? About half of the NO2 will turn into N2O4 2. Test: Click Play. Select the BAR CHART tab and check that Moles is selected. Observe the bar chart for about 30 seconds. As time goes by, what do you notice about the bars representing moles NO2 and moles N2O4? The bar representing NO2 goes down and the bar representing N2O4 goes up. 3. Observe: Click Pause ( ). Select the GRAPH tab. Click the (–) zoom control on the horizontal axis until you can see the whole graph. What do you notice? The amount of NO2 is double the amount of N2O4, This situation, in which the overall amounts of reactants and products does not change significantly over time, is called a chemical equilibrium. 4. Record: On the BAR CHART tab, turn on Show data values. How many moles of NO2 and N2O4 are there right now? Moles NO2 4.36 Moles N2O4 1.82 5. Calculate: Suppose all the NO2 molecules were synthesized into N2O4. Given the equation 2NO2 ⇄ N2O4, how many moles of N2O4 would be produced? 4 [Show More]
Last updated: 1 year ago
Preview 1 out of 10 pages
Instant download
Buy this document to get the full access instantly
Instant Download Access after purchase
Add to cartInstant download
Reviews( 0 )
Document information
Connected school, study & course
About the document
Uploaded On
May 14, 2021
Number of pages
10
Written in
Additional information
This document has been written for:
Uploaded
May 14, 2021
Downloads
0
Views
94