Maywood High SchoolCHE 1111GIZMO Reaction Energy Answers.
Document Content and Description Below
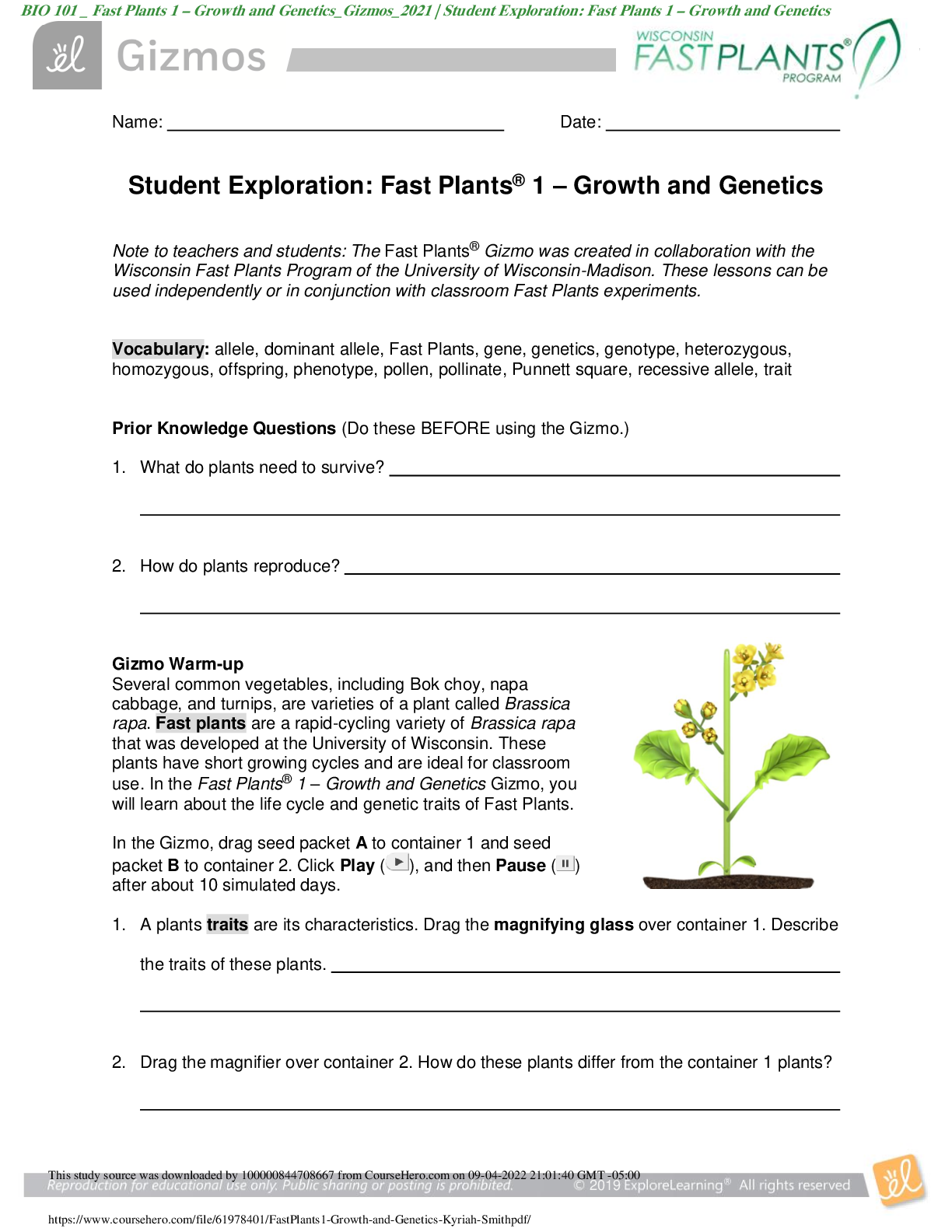
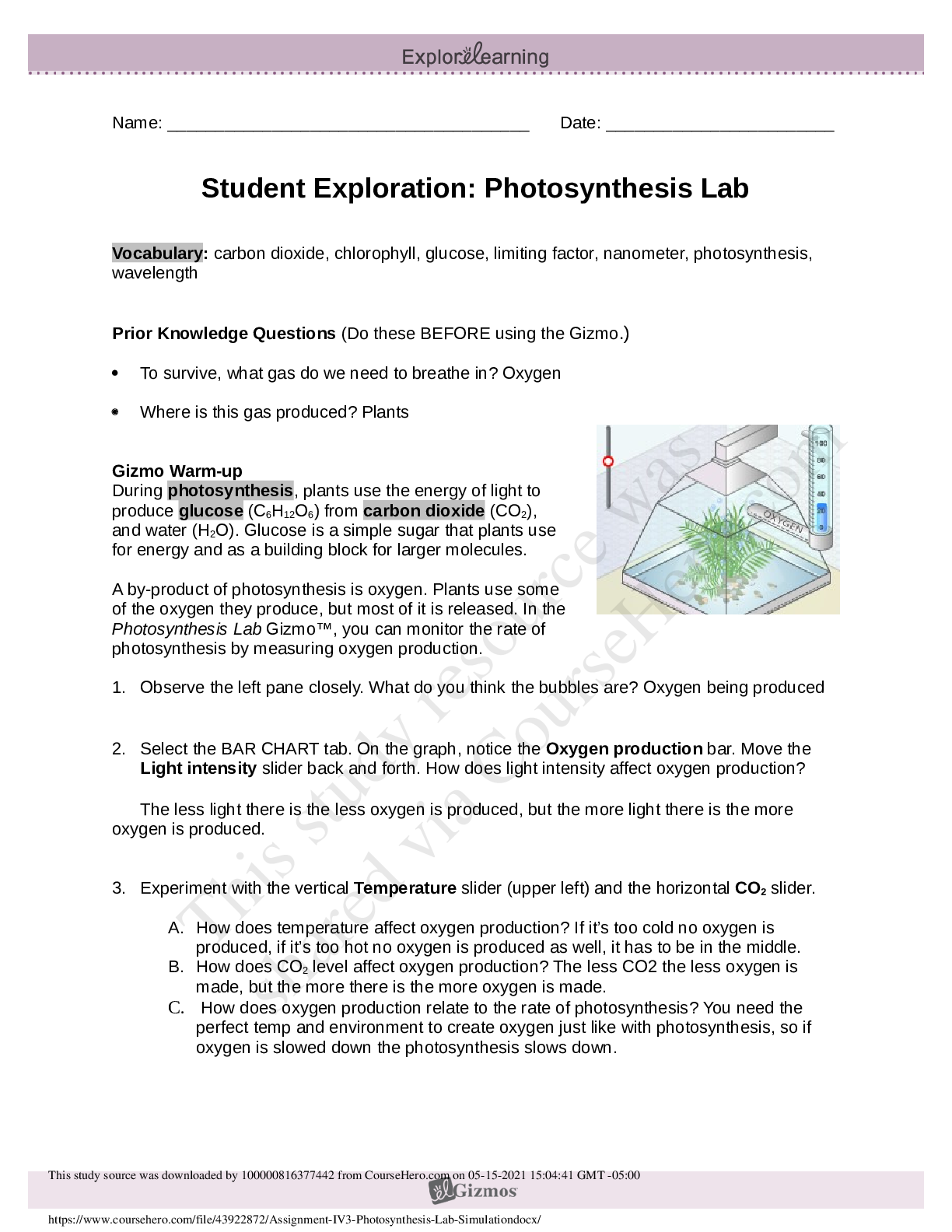
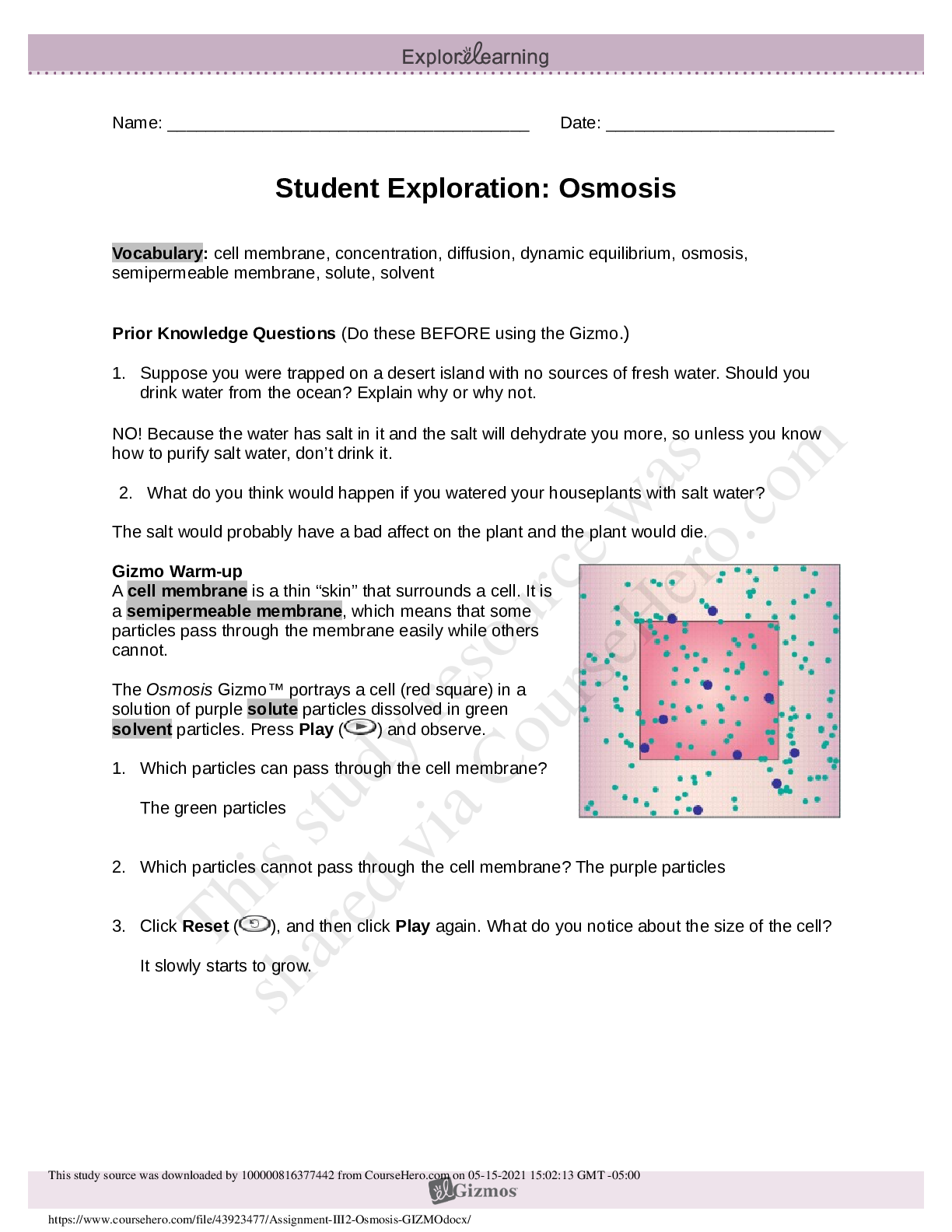
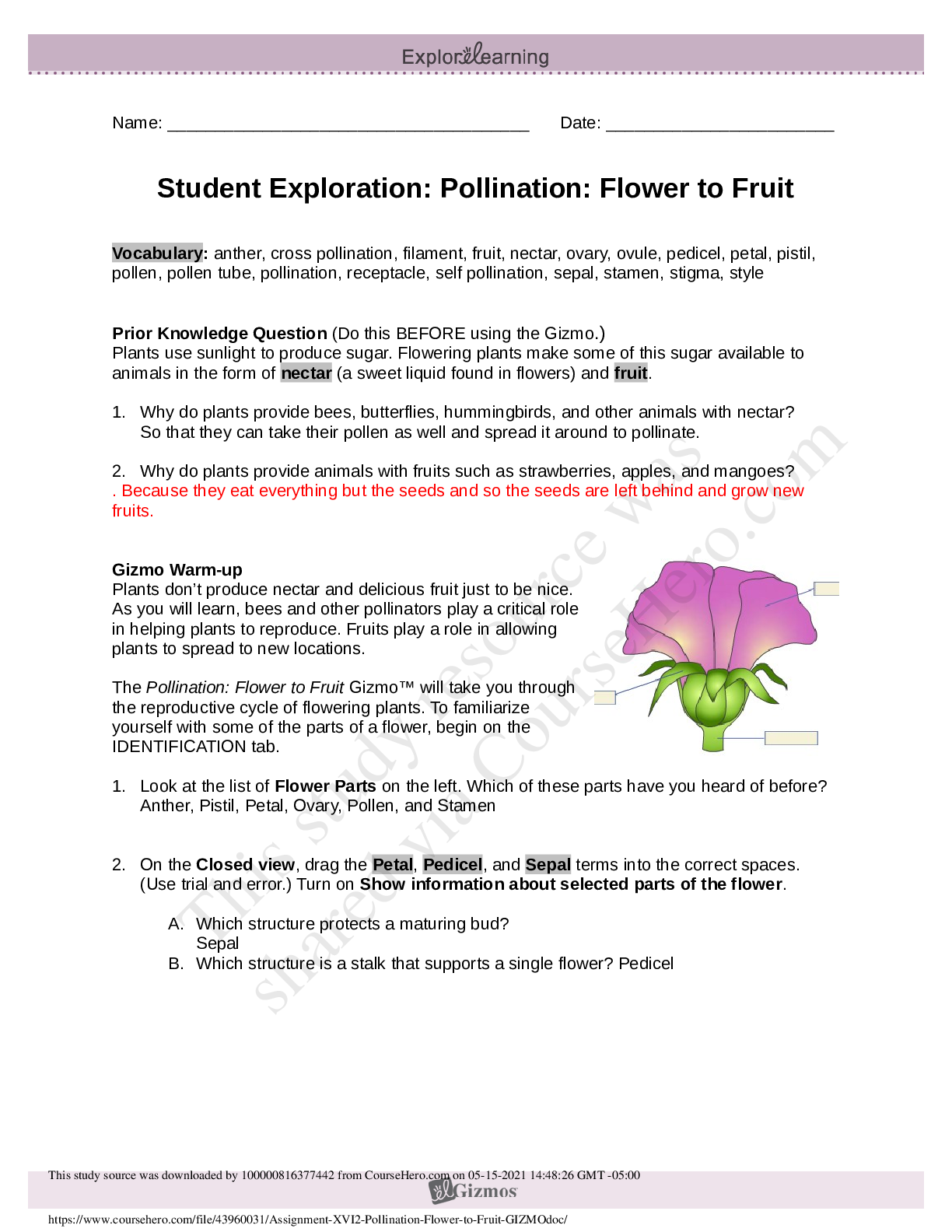
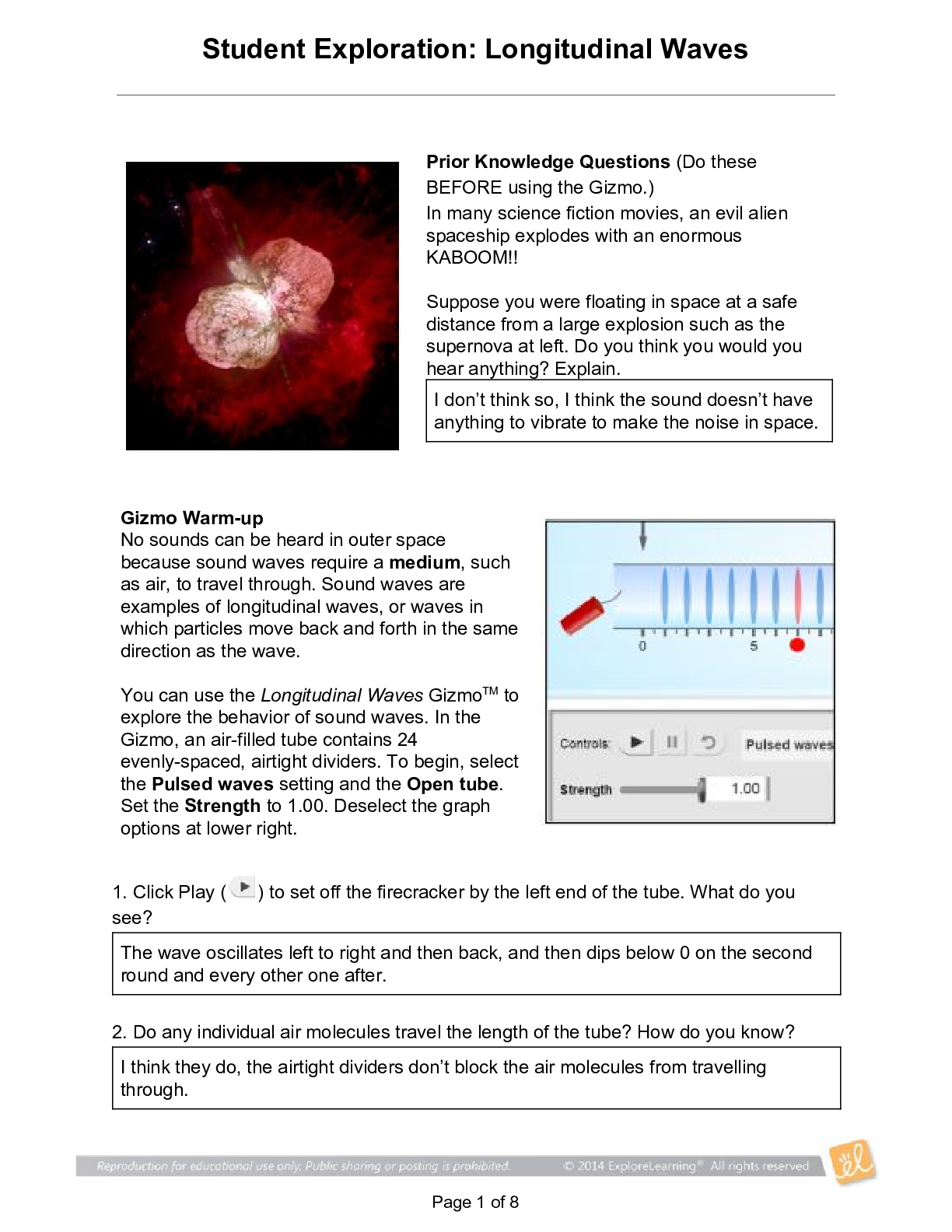
1. Two magnets are stuck together. What might you have to do to get them to separate? Pull them apart 2. Suppose you held two magnets a short distance apart, then let go. What would happen? Depends... on which poles of the magnets were facing each other. If one was positive and one was negative, they would be attracted to each other. If they were either both positive or negative, they would repel. 3. Think about the magnets in terms of energy. In which case do you increase the potential energy of the magnets? In which case do you increase the kinetic energy of the magnets? Kinetic energy increases if you were to move magnets with the same charge towards each other because they are going to repel. Potential energy would increase if they were different poles because they are attracted to each other. Gizmo Warm-up Just like magnets, atoms of different elements are attracted together to form chemical bonds. Breaking these bonds requires energy. When a new bond forms, energy is released and temperatures rise. In the Reaction Energy Gizmo, you will explore how the energy of chemical bonding relates to temperature changes that occur during chemical reactions. To begin, check that Reaction 1 and Forward are selected. In this reaction, hydrogen (H2) and oxygen (O2) react to form water (H2O). The reaction takes place inside a device called a calorimeter. Inside the calorimeter, a small chamber holds the reactants. The rest of the calorimeter is filled with water. 1. Click Play ( ). What happens? Temperature increases, moving molecules. 2. How does the temperature change? Increases Introduction: The heat energy stored in a chemical system is called the enthalpy (H) of the system. When atoms are joined by a chemical bond, energy must be added to pull them apart. This increases the enthalpy of the system. When a chemical bond forms, energy is released as shared electrons move into lower-energy orbitals. This causes the enthalpy to decrease. Question: How can you predict how much energy is released in a chemical reaction? 1. Predict: In the warm-up activity, you observed how the reaction inside the chamber affected the temperature of the surrounding water. Based on what happens to the surrounding water, do you think heat energy (enthalpy) is absorbed in the reaction or released? Explain. Heat is released because the surrounding water is absorbing the heat (which we know because the temperature increases). 2. Observe: In the Gizmo, the energy required to break a chemical bond is modeled by placing a molecule into a set of mechanical claws. Place one of the hydrogen (H2) molecules between the claws, and press the Break bond. A. What happens? The bond between the atoms is broken and energy is absorbed. B. Look under the Energy absorbed column of the table. How much energy was required to break this bond? 436kJ/mol Note: The energy is given here in units of kilojoules per mole (kJ/mol). This is the energy, in kilojoules, required to break all of the H–H bonds in one mole of H2 gas. C. Remove the hydrogen atoms from the claws and then break apart the other H–H molecule. What is the total energy absorbed so far? 872kJ/mol 3. Measure: Notice that the oxygen atoms are connected by a double covalent bond. This is because the oxygen atoms share two pairs of electrons. Place the oxygen molecule in the claws and press the Break bond. A. How much energy is required to break the first O–O bond? 349kJ/mol Reproduction for educational use only. Public sharing or posting prohibited. © 2020 ExploreLearning™ All rights reserved d. How much energy is needed to break both bonds? 495kJ/mol C. What is the total energy required to break up two moles of H2 molecules and one mole of O2 molecules? 1367kJ/mol 4. Create: Remove the two oxygen atoms from the claws. Now the claws disappear and you see a template for creating a water molecule. Drag an oxygen and a hydrogen atom into the template. (If necessary, use the Key on the right-hand side as a reference.) A. Click Create bond. What happens? New bond is formed; energy is released. B. The “jiggling” animation you see represents the release of kinetic energy that occurs when a bond is formed. How much energy was released? 463kJ/mol C. Drag another hydrogen molecule into the template and click Create bond to make a water molecule. What is the total energy released so far? 926kJ/mol D. Drag the first water molecule away from the template, then use the Gizmo to create a second water molecule. What is the total energy released now? 1852kJ/mol 5. Calculate: Compare the energy absorbed in breaking up the molecules to the energy released when new bonds are formed. A. In this reaction, was more energy absorbed or released? Released B. How does this relate to the change in temperature observed for this reaction? Both reactions were shown to be releasing heat/energy. C. The change in enthalpy (∆H) of the system is equal to the total energy absorbed minus the total energy released. What is the ∆H value for this reaction? 1367kJ/mol - 1852kJ/mol = -485kJ/mol Compare this value to the Theoretical ∆H listed on the right side. 6. Draw conclusions: The experimental ∆H value was determined by measuring how much heat the reaction produced inside the calorimeter. This is calculated based on the temperature change of the reaction, the Reproduction for educational use only. Public sharing or posting prohibited. © 2020 ExploreLearning™ All rights reserved [Show More]
Last updated: 1 year ago
Preview 1 out of 8 pages

Reviews( 0 )
Document information
Connected school, study & course
About the document
Uploaded On
May 20, 2021
Number of pages
8
Written in
Additional information
This document has been written for:
Uploaded
May 20, 2021
Downloads
0
Views
70











.png)