Anatomy > STUDY GUIDE > West Coast University ANAT 260 Physiology PGA 2 Study Guide for Chapters 4-6. (All)
West Coast University ANAT 260 Physiology PGA 2 Study Guide for Chapters 4-6.
Document Content and Description Below
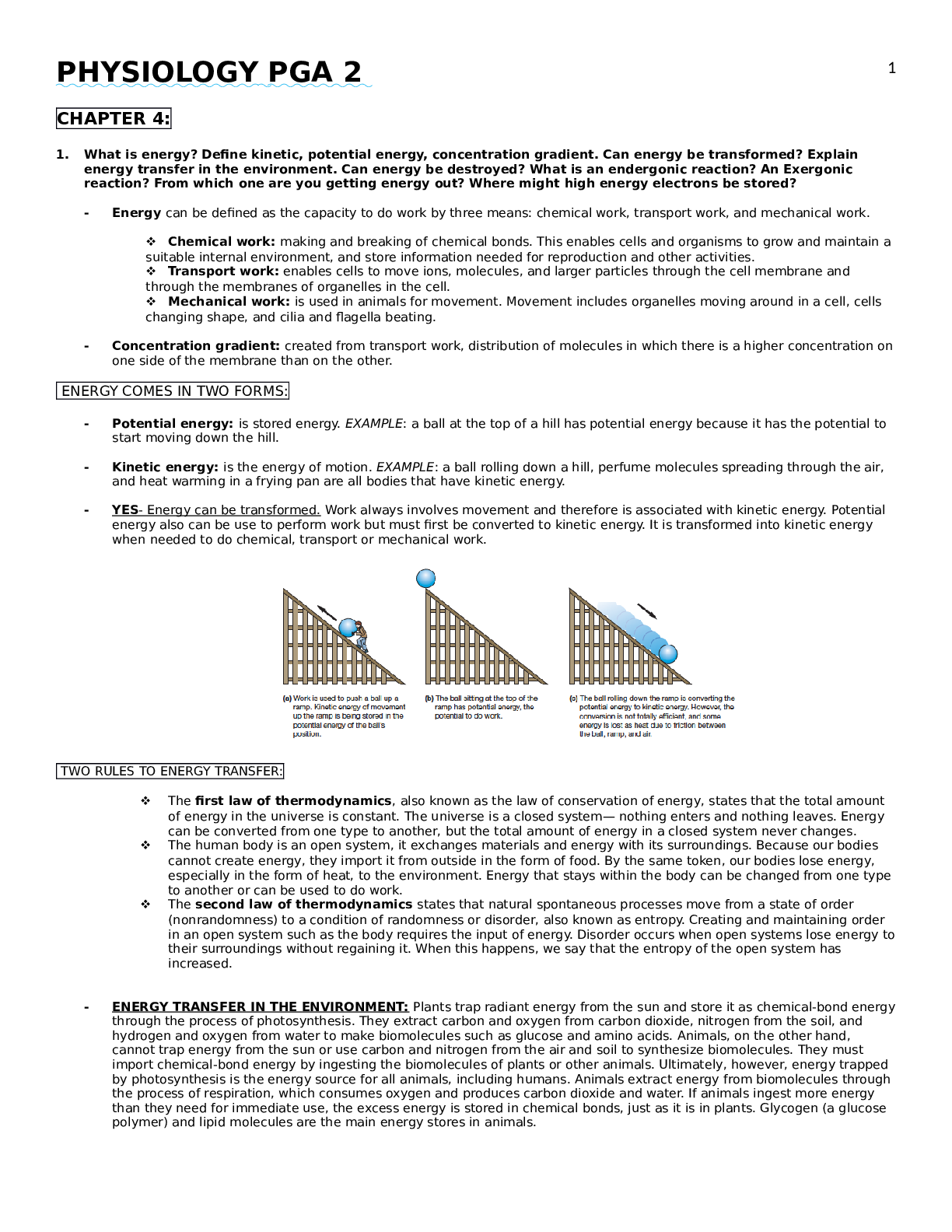
PHYSIOLOGY PGA 2 CHAPTER 4: 1. What is energy? Define kinetic, potential energy, concentration gradient. Can energy be transformed? Explain energy transfer in the environment. Can energy be destroy... ed? What is an endergonic reaction? An Exergonic reaction? From which one are you getting energy out? Where might high energy electrons be stored? - Energy can be defined as the capacity to do work by three means: chemical work, transport work, and mechanical work. Chemical work: making and breaking of chemical bonds. This enables cells and organisms to grow and maintain a suitable internal environment, and store information needed for reproduction and other activities. Transport work: enables cells to move ions, molecules, and larger particles through the cell membrane and through the membranes of organelles in the cell. Mechanical work: is used in animals for movement. Movement includes organelles moving around in a cell, cells changing shape, and cilia and flagella beating. - Concentration gradient: created from transport work, distribution of molecules in which there is a higher concentration on one side of the membrane than on the other. ENERGY COMES IN TWO FORMS: - Potential energy: is stored energy. EXAMPLE: a ball at the top of a hill has potential energy because it has the potential to start moving down the hill. - Kinetic energy: is the energy of motion. EXAMPLE: a ball rolling down a hill, perfume molecules spreading through the air, and heat warming in a frying pan are all bodies that have kinetic energy. - YES- Energy can be transformed. Work always involves movement and therefore is associated with kinetic energy. Potential energy also can be use to perform work but must first be converted to kinetic energy. It is transformed into kinetic energy when needed to do chemical, transport or mechanical work. TWO RULES TO ENERGY TRANSFER: The first law of thermodynamics, also known as the law of conservation of energy, states that the total amount of energy in the universe is constant. The universe is a closed system— nothing enters and nothing leaves. Energy can be converted from one type to another, but the total amount of energy in a closed system never changes. The human body is an open system, it exchanges materials and energy with its surroundings. Because our bodies cannot create energy, they import it from outside in the form of food. By the same token, our bodies lose energy, especially in the form of heat, to the environment. Energy that stays within the body can be changed from one type to another or can be used to do work. The second law of thermodynamics states that natural spontaneous processes move from a state of order (nonrandomness) to a condition of randomness or disorder, also known as entropy. Creating and maintaining order in an open system such as the body requires the input of energy. Disorder occurs when open systems lose energy to their surroundings without regaining it. When this happens, we say that the entropy of the open system has increased. - ENERGY TRANSFER IN THE ENVIRONMENT: Plants trap radiant energy from the sun and store it as chemical-bond energy through the process of photosynthesis. They extract carbon and oxygen from carbon dioxide, nitrogen from the soil, and hydrogen and oxygen from water to make biomolecules such as glucose and amino acids. Animals, on the other hand, cannot trap energy from the sun or use carbon and nitrogen from the air and soil to synthesize biomolecules. They must import chemical-bond energy by ingesting the biomolecules of plants or other animals. Ultimately, however, energy trapped by photosynthesis is the energy source for all animals, including humans. Animals extract energy from biomolecules through the process of respiration, which consumes oxygen and produces carbon dioxide and water. If animals ingest more energy than they need for immediate use, the excess energy is stored in chemical bonds, just as it is in plants. Glycogen (a glucose polymer) and lipid molecules are the main energy stores in animals. [Show More]
Last updated: 1 year ago
Preview 1 out of 11 pages
Instant download
Buy this document to get the full access instantly
Instant Download Access after purchase
Add to cartInstant download
Reviews( 0 )
Document information
Connected school, study & course
About the document
Uploaded On
Jun 08, 2021
Number of pages
11
Written in
Additional information
This document has been written for:
Uploaded
Jun 08, 2021
Downloads
0
Views
44

















.png)

.png)

