*NURSING > Lab Experiment > CHEM-120 Unit 3 Lab: OL Lab 6: Solution Preparation: From Salt to Solution (All)
CHEM-120 Unit 3 Lab: OL Lab 6: Solution Preparation: From Salt to Solution
Document Content and Description Below
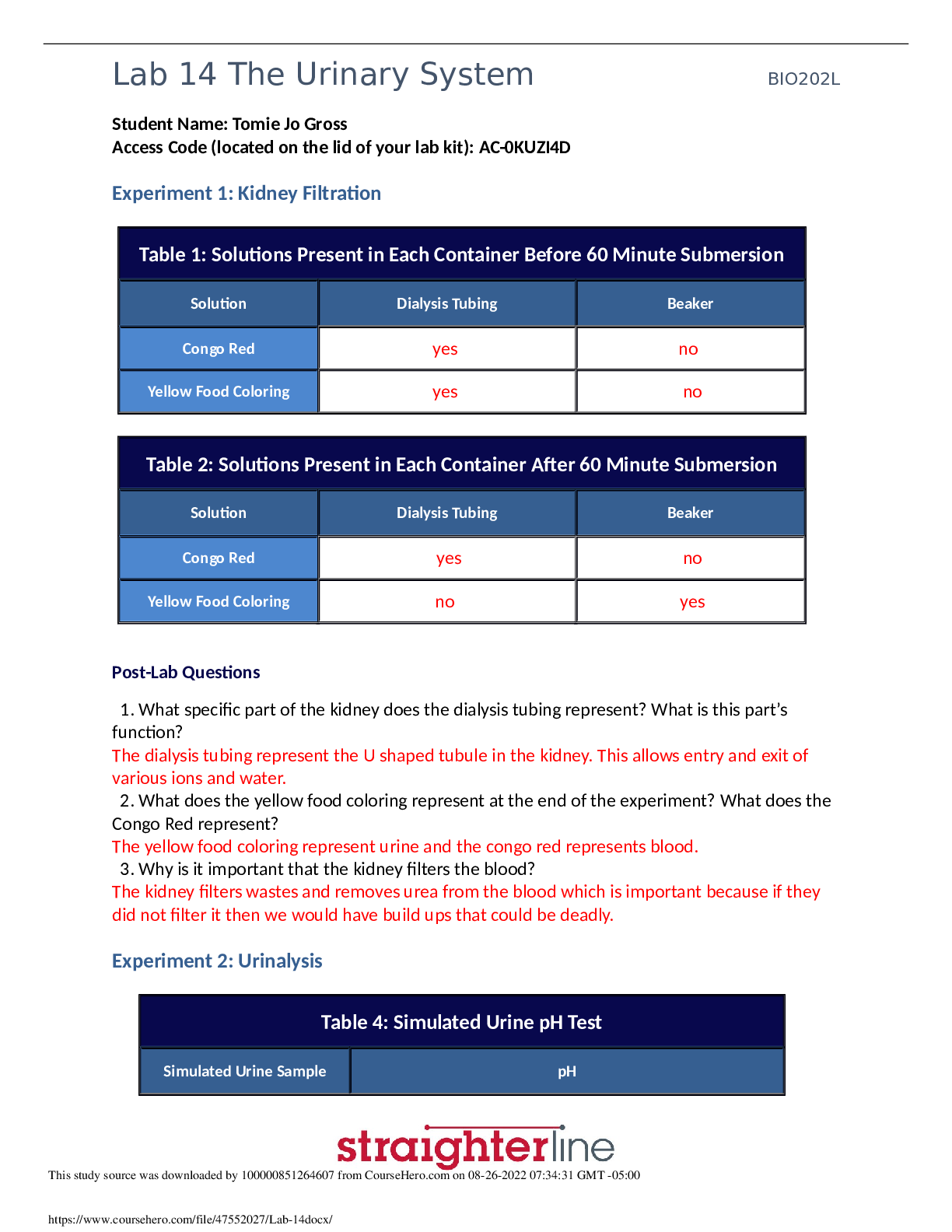
Learning Objectives: Prepare an aqueous solution of a specific concentration from a pure salt Correctly use an analytical balance, a volumetric pipette, a volumetric flask, and a measuring ... cylinder Explain the relationship between molarity and mass concentration Introduction Prepare to become a solution champion! In this simulation, you will complete all the steps involved in preparing an aqueous solution of a given molarity from ammonium chloride – a water-soluble salt. Use that balance Your first mission will be to determine the exact amount of ammonium chloride needed for your solution. When the calculations are completed, you will move to your first workbench, where you’ll find an analytical balance. You will use this high precision instrument to obtain just the right amount of the substance. It’s a tricky procedure, but you’ll have unlimited time and attempts to get it right. Glassware and quantitative transfer Moving on to the second workbench, you will explore and determine what glassware will be appropriate for you to use. Your lab guide and mentor, Dr. One, will walk you through the process of preparing the solution. You will have the freedom to use the equipment as you like, but only by following Dr. One’s instructions will you be successful in making the right solution. To make sure you can always redo the essential steps of the process, Dr. One will provide you with a fantastic reset button, so you can try again if you make a mistake. Solve the preparation In the end, only by carefully ensuring that the right amount of water and ammonium chloride is used can you make the correct solution. Are you prepared for solving how to perform a Solution Preparation? Part 1: Complete the Labster Lab: Solution Preparation: From salt to solution Part 2: Report and Reflection Purpose: Describe in complete sentences and in your own words, the purpose of this experiment. The purpose of this experiment was to explore the relationship between molarity and mass concentration by preparing an aqueous NaCl solution with a certain concentration. The simulation also explains how to correctly use a balance and why volumetric pipettes allow for the highest accuracy in concentration preparation. Observations: Record thr [Show More]
Last updated: 1 year ago
Preview 1 out of 4 pages

Reviews( 0 )
Document information
Connected school, study & course
About the document
Uploaded On
Aug 23, 2021
Number of pages
4
Written in
Additional information
This document has been written for:
Uploaded
Aug 23, 2021
Downloads
0
Views
115













.png)


.png)











.png)