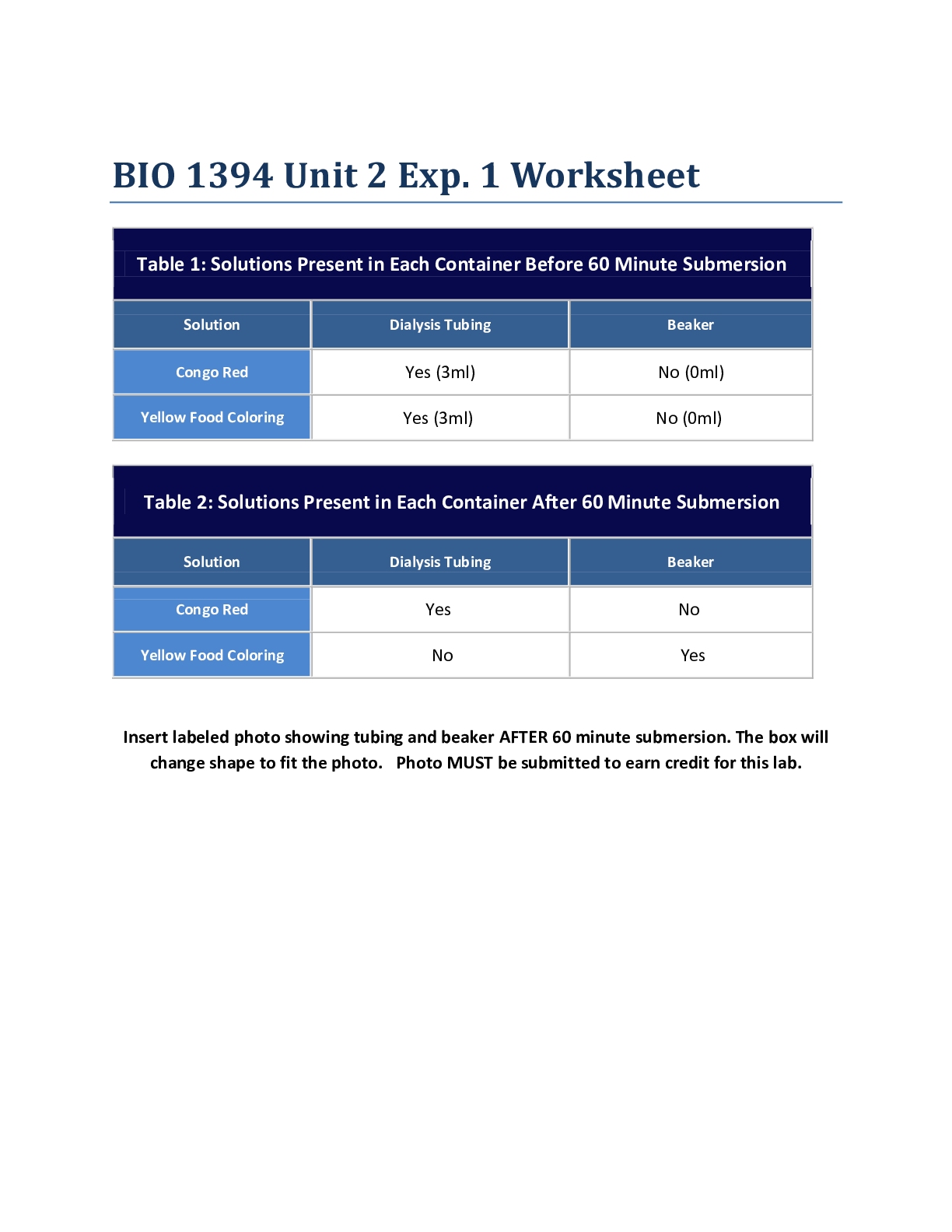
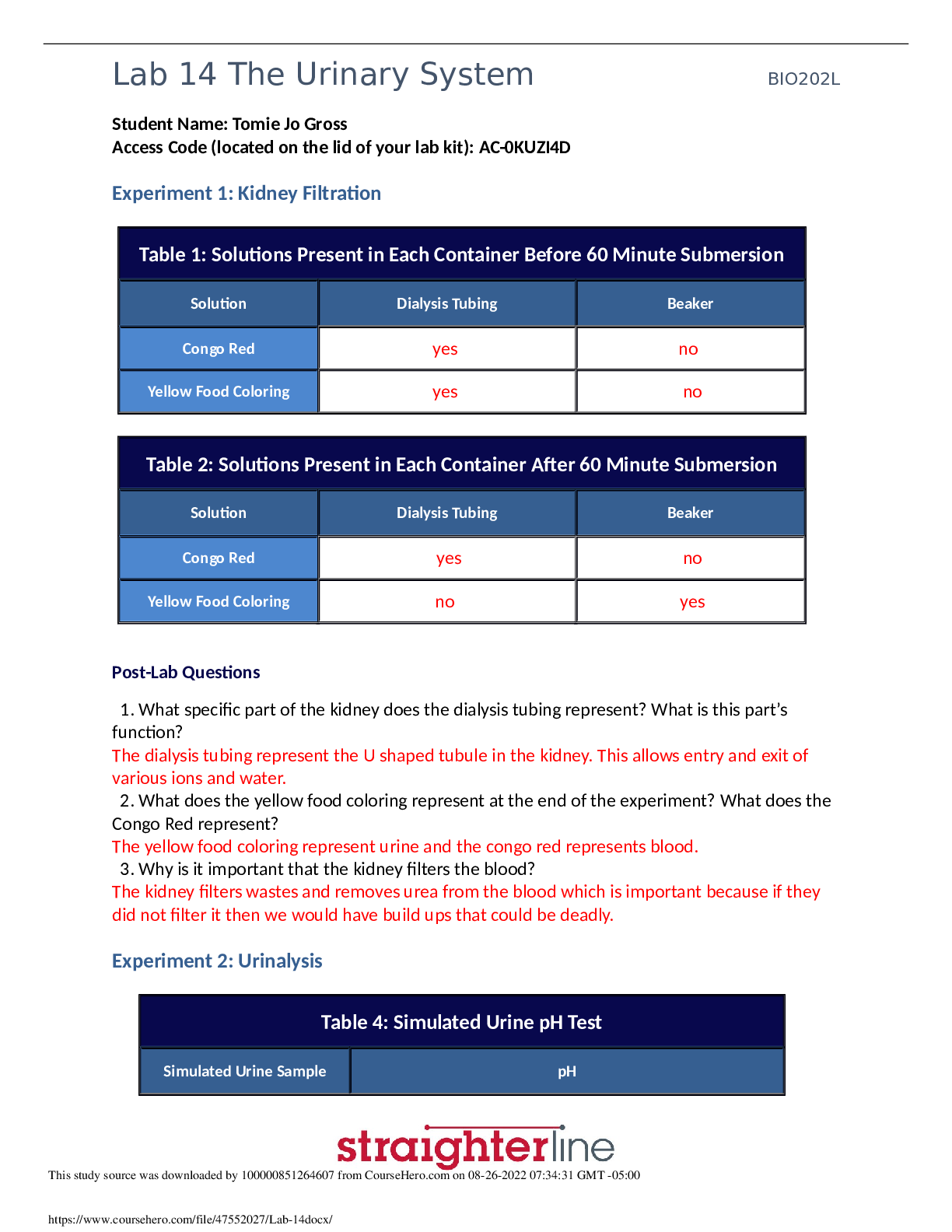
Chemistry > Lab Experiment > Lab Experiment > CHEMISTRY 11101 Lab Report#2: this lab was to estimate Avogadro’s number using st (All)
Lab Experiment > CHEMISTRY 11101 Lab Report#2: this lab was to estimate Avogadro’s number using stearic acid.
Document Content and Description Below
Introduction: The purpose of this lab was to estimate Avogadro’s number using stearic acid. We dropped a precise amount of stearic acid onto the surface of water using a well-calibrated pipette, e... nough to form a monolayer “cake” of molecules onto the surface of the water. The polar head of the stearic acid molecule and the non-polar end, which sticks away from the water, allows a monolayer to form on the surface of water. From the volume required to form a monolayer, the number of drops of stearic acid was counted. The thickness of the layer was then used to compute the volume of a singular carbon atom, and this value was then used to determine the volume of a mole of carbon atoms used. From this value, we were finally able to estimate Avogadro’s number. Experimental Methods: A 15cm watch glass was cleaned with detergent solution and rinsed with both cold and distilled water. The watch glass was then dried and placed on plain piece of white paper. 10mL of hexane was poured into a 50mL beaker. A plastic pipette was used to place drops of stearic acid into a graduated cylinder. The number of drops required to deliver a volume of 1.00 mL was averaged from four trials. A 10mL graduated cylinder was filled with 1.0 mL of n-hexane. The number of drops of n-hexane needed to reach the next mL was counted, repeated twice, and averaged so the error was within The diameter of the watch glass was measured within 0.1cm accuracy and repeated twice. The watch glass was filled with distilled water. Stearic acid with a concentration of 0.1480 g/L was poured into a 10mL graduated cylinder. The plastic pipette was rinsed with n-hexane and then filled with stearic acid. The drops of stearic acid placed onto the surface of the water in the watch glass was counted, while the pipette remained vertical. Once a drop’s form persisted for approximately 30 seconds, one more drop than necessary to form the monolayer was added to the surface of water. The pipette was used to remove some of the solution from the watch glass. The remaining solution was poured into a 600mL beaker, and the watch glass was cleaned with soap water, tap water, and distilled water. This procedure to drop the stearic acid on the water to form a monolayer was repeated two times. [Show More]
Last updated: 1 year ago
Preview 1 out of 5 pages

Reviews( 0 )
Document information
Connected school, study & course
About the document
Uploaded On
Oct 14, 2020
Number of pages
5
Written in
Additional information
This document has been written for:
Uploaded
Oct 14, 2020
Downloads
0
Views
115


.png)