Chemistry > Lab Experiment > CHEMI 1105 Doll _Chem1105 Experiment 8: Oxidation-Reduction Activity Series. All 10 Post-Lab Questio (All)
CHEMI 1105 Doll _Chem1105 Experiment 8: Oxidation-Reduction Activity Series. All 10 Post-Lab Questions: Answered.
Document Content and Description Below
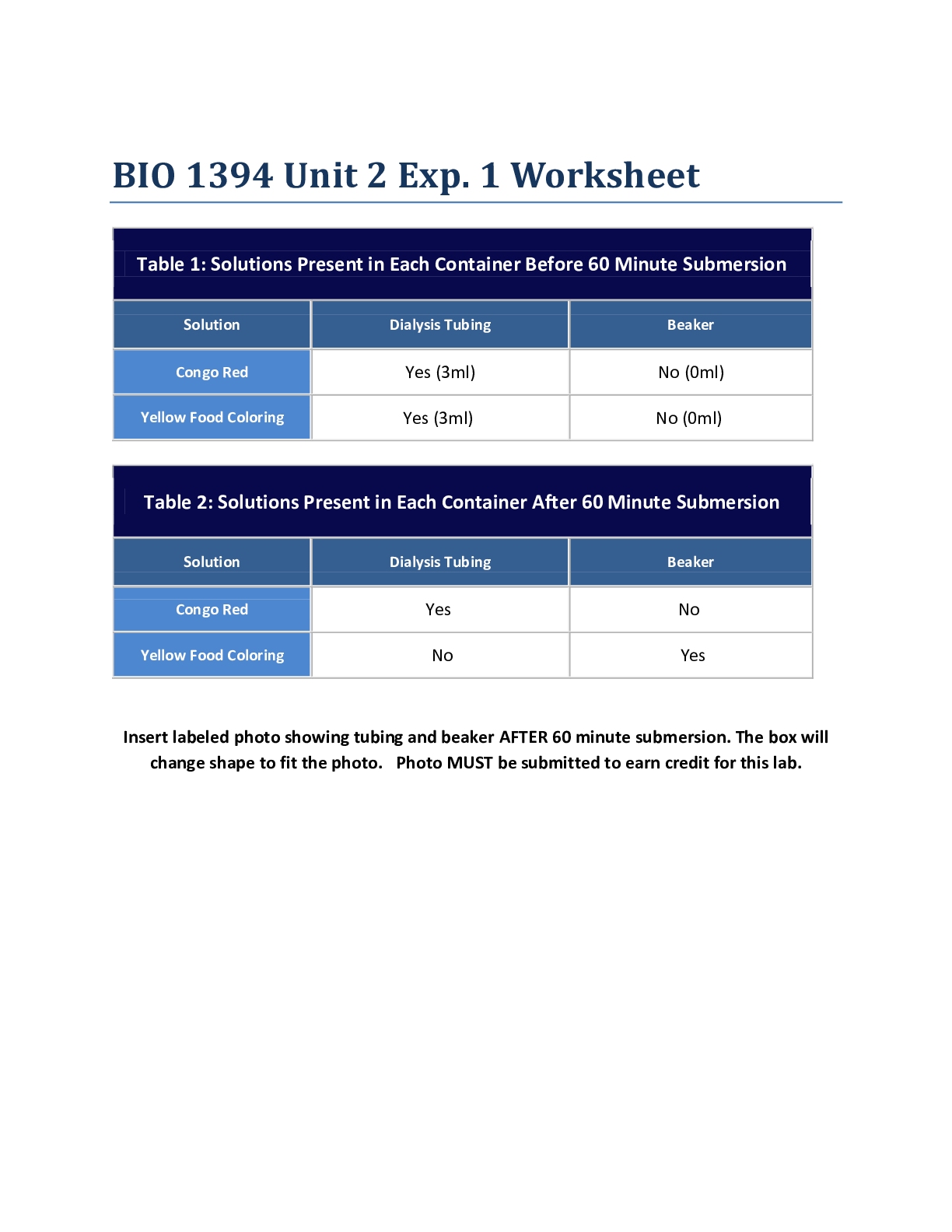
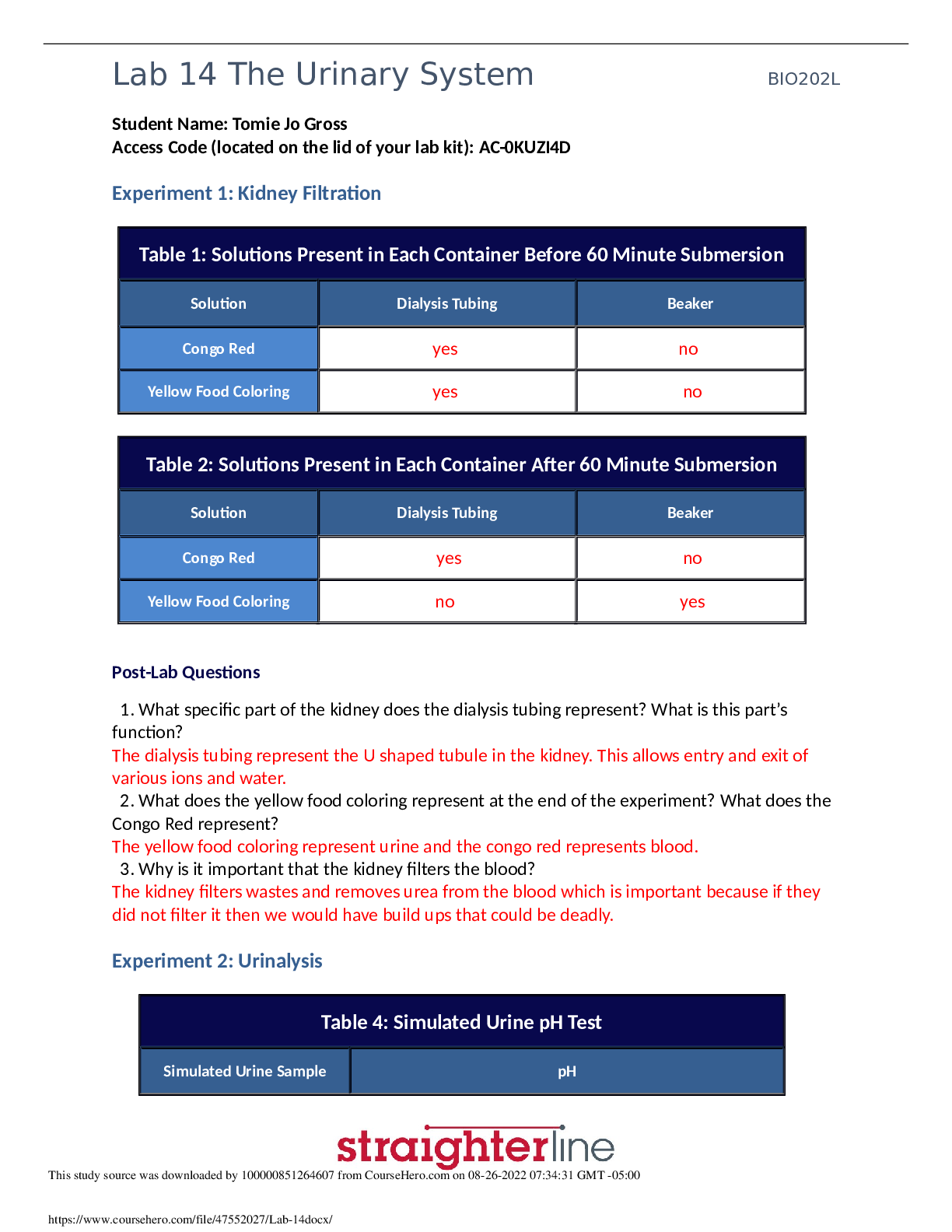
Purpose: The purpose of this lab was to let us work closely with oxidation-reduction reactions. We got to see how different chemicals reacted and practiced figuring out which metals and solutions we... re reducing agents, oxidizing agents, and spectator ions, and got to practice creating an activity series. Procedure: Exercise 1: Describing an Oxidation-Reduction Reaction 1. You must wear your goggles and gloves for BOTH exercises. Put them on. 2. Get a test tube, silver nitrate (AgNO3) solution, and copper foil pieces (Cu), as well as the plastic tweezers. Metal tweezers could react with other metals in the experiment. 3. Record observations of appearances of metals and solutions in Data Table 1. 4. Use tweezers to add 2 pieces of Cu to the test tube. 5. Add all of the AgNO3 bottle to test tube. Throw away empty bottle. 6. Do not allow silver nitrate to get onto your skin. 7. Observe reaction for 1 minute. Take down observations in Data Table 1. 8. Allow reaction to go on for 30 minutes and record appearance of reaction in Data Table 1. 9. Balance the equation for the redox reaction. Include oxidation numbers as well as the total charge contributions for the elements involved in the reaction. 10. Identify which element is oxidized and which element is reduced. Identify spectator ion. Record in Data Table 1. 11. Identify which element was the oxidizing agent and determine which element was the reducing agent. Record in Data Table 1. 12. Clean up. Exercise 2: Creating an Activity Series 1. Get a 24 well plate. 2. Locate copper foil and lead (II) nitrate pipet. 3. Locate copper foil pieces and plastic tweezers. Put a piece of copper in well A1. 4. Snip off tip of lead (II) nitrate with scissors and add 15 drops to well A1. 5. Observe for 1 minute and record in Data Table 2. 6. Note what time it is, because you will make observations after 30 minutes. 7. Snip off tip of zinc nitrate pipet with scissors and add 15 drops to well A2. Observe well A2 for 1 minute and record immediate sign s of chemical reaction in Data Table 2. 9. Get 2 lead squares and a paper towel. Use tweezers to place 2 pieces of lead on paper towel. 10. Use pocket knife to scrape surface of the lead, removing rust that might have accumulated. Keep on paper towel because lead shavings can be hazardous. Scrape surface until it is shiny and wipe each square with paper towel to get rid of shavings. 11. Place lead square in well B1 and B2. 12. Throw out paper towel and lead particles. Wash knife with soap and water. 13. Take off gloves and place in trash inside out. Wash hands and dry. 14. Get a new pair of gloves and put them on. 15. Snip off tip of copper (II) sulfate pipet and add 15 drops into well B1. Record observations after 1 minute and record in Data Table 2. 16. Add 15 drops Zn(NO3)2 to well B2. After 1 minute, record observations in Data Table 2. 17. Get 2 pieces of mossy zinc (Zn) and place in wells C1 and C2. 18. Add 15 drops of CuSO4 to well C1. Record observations after 1 minute in Data Table 2. 19. Add 15 drops of Pb(NO3)2 to well C2. Record observations after 1 minute. 20. Let 30 minutes pass and record observations for all reactions in Data Table 2. 21. Determine if a chemical reaction occurred in each well. In Data Table 3, record yes or no. 22. In 3, if you answered yes for any instances, write a balanced chemical equation. 23. Clean up. Data and Observations: Data Table 1: Redox Reaction of Copper and Silver Nitrate Initial Observations: before beginning Copper: Thin like tin foil, shiny copper colored, flimsy Silver Nitrate: clear liquid Observations Copper is wilting and turning a greenishblack color. crystals forming on copper foil Observations after 30 minutes Copper has completely crystallized—it is now a white ball at the bottom of the test tube Silver Nitrate has a slightly blue tint to it Chemical equation Cu(s) + 2AgNO3(aq) 2Ag(s) + Cu(NO3)2(aq) Element that is oxidized Copper (Cu) Element that is reduced Silver (Ag) Spectator ion Nitrate (NO3) Oxidizing agent Silver (Ag) Post-Lab Questions: 1. Define oxidation, reduction, and oxidation number. Describe how oxidation and reduction affect the oxidation number of an element Define oxidizing agent, reducing agent, and spectator ion In the reaction of copper and silver nitrate, a new substance appeared in the test tube. Describe the physical appearance of the substance and identify its chemical formula Given an activity series in which the most active metals are at the top of the list and the least active metals are at the bottom of the list, would copper be listed above silver or would silver be listed above copper? Support your answer Solid copper sulfide and silver nitrate react to form copper (II) nitrate and solid silver sulfide. Write a balanced equation that describes the reaction. Identify the oxidation number of each element in the reaction. (You do not need to include the total contribution of charge.) Is this reaction a redox reaction or a non-redox reaction? Explain. List each of the metals tested in Exercise 2. Indicate the oxidation number when each element is pure and the oxidation number when each element is in a compound Which of the metals in Exercise 2 was the strongest oxidizing agent? Was there an instance when this metal also acted as the reducing agent? Explain Which of the metals in Exercise 2 was the strongest reducing agent? Was there an instance when this metal also acted as an oxidizing agent? Explain your answer How does ease of oxidation correlate with activity? Do highly active metals tend to donate electrons or accept electrons from other metalsCreate an activity series for copper, lead, and zinc. Place the most active metal at the top of the list. [Show More]
Last updated: 1 year ago
Preview 1 out of 7 pages
.png)
Reviews( 0 )
Document information
Connected school, study & course
About the document
Uploaded On
Apr 21, 2021
Number of pages
7
Written in
Additional information
This document has been written for:
Uploaded
Apr 21, 2021
Downloads
0
Views
55