Exploring Density Lab
Document Content and Description Below
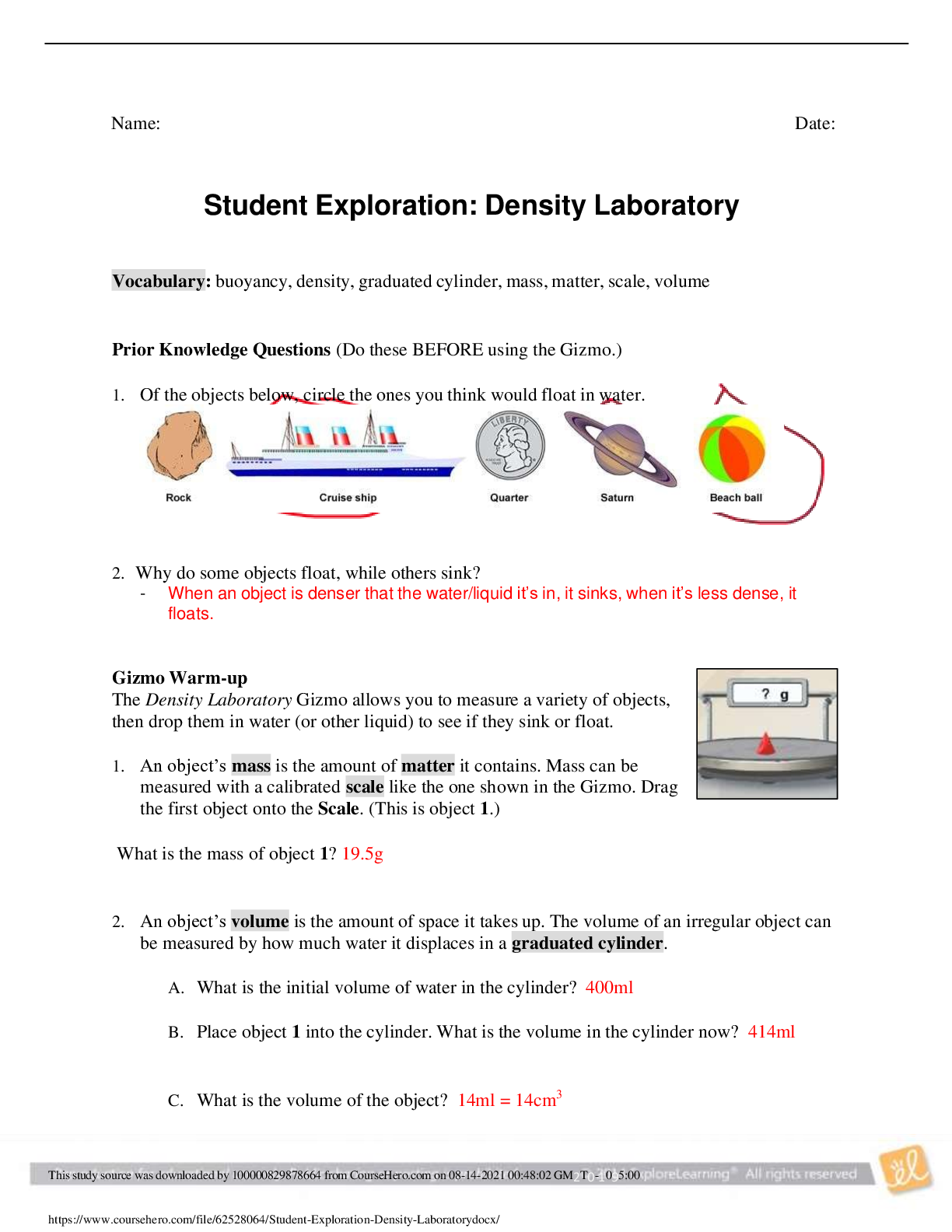
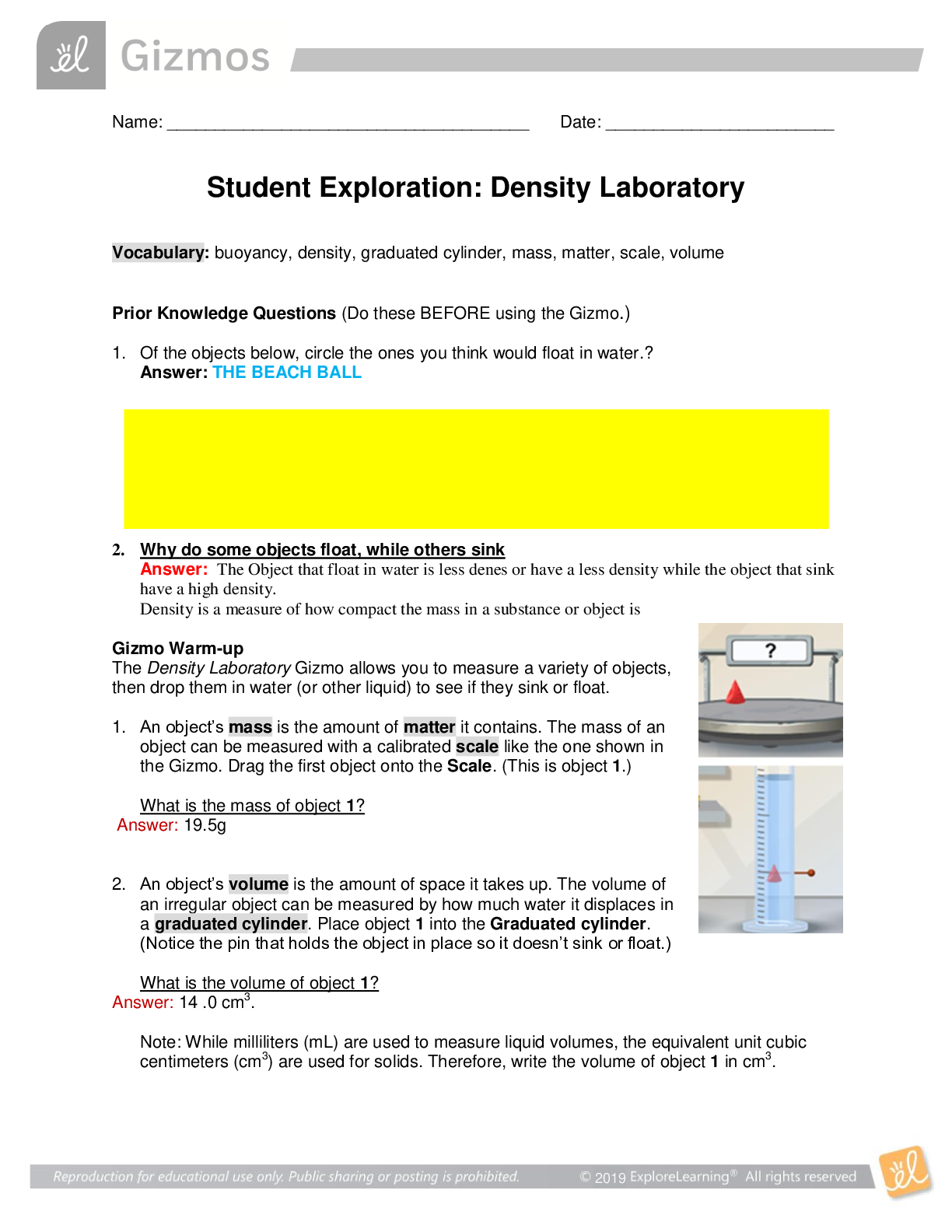
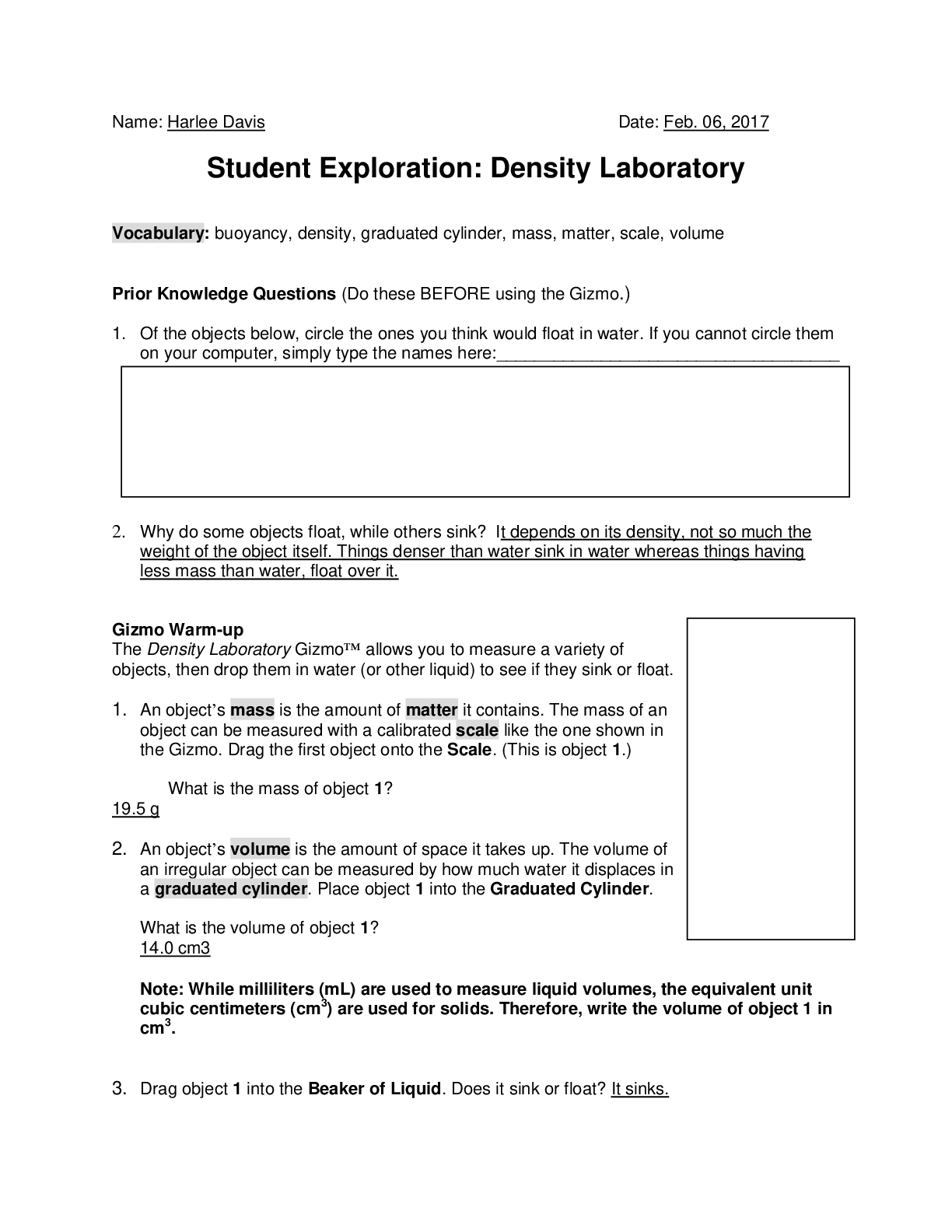
Exploring Density Investigation Manual EXPLORING DENSITY Table of Contents 2 Overview 2 Objectives 2 Time Requirements 3 Background 4... Materials 5 Safety 6 Preparation 7 Activity 1 8 Activity 2 11 Activity 3 12 Activity 4 13 Disposal and cleanup Overview Explore the concept of density through four activities. In the first activity, the densities of three regular solids will be determined. In the second activity, the densities of water and sucrose solutions will be determined from graphs of mass versus volume. Using the information collected in the first two activities, you will predict and test if the solids sink or float in the sucrose solutions. In the final activity, the density of a beverage will be used to calculate the concentration of sucrose. Objectives • Calculate the densities of regular solids. • Determine the densities of solutions by plotting mass versus volume. • Predict whether objects will sink or float in different solutions based on the densities of the solutions and objects. • Determine the concentration of sucrose in a beverage based on a graph of known sucrose concentration densities. Time Requirements Preparation 30 minutes Activity 1 15 minutes Activity 2 60 minutes Activity 3 15 minutes Activity 4 30 minutes The lab may be paused at any time and continued later. If the lab is paused prior to completion, ensure that all liquids are sealed to prevent evaporation. Key Personal protective equipment (PPE) ! gloves goggles apron video camera stop timer warning watch Background Density Density is a physical property of matter that is based on mass and volume of the substance. Mass and volume are extensive properties, meaning that they are dependent on the quantity of matter. However, density is an intensive prop- erty and does not change with quantity. Density is a derived unit, meaning that it is composed of two basic units. Density can be defined as mass per unit of volume. The mathematical expres- sion for density is D = M/V, in which mass (M) is expressed in grams (g), and volume (V) is expressed in cubic centimeters (cm3). Milliliter (mL) units may replace cm3 when describing liquids, because the two units are fundamentally equivalent. Density and weight are not the same value. For instance, some people might say that steel weighs more than sand. However, a huge mound of sand obviously weighs more than one steel marble. It would be more accurate to compare masses of steel and sand that have equal volumes. In doing so, they would find that 1 cm3 of steel has a greater mass than that of 1 cm3 of sand. In other words, steel does not weigh more than sand; rather, steel is more denser than sand. Best-Fit Line A best-fit line (or regression line) is a line within a scatter plot that shows the linear relationship of data. A best-fit line allows data points that were not collected during an experiment to be predicted. Most experiments have some exper- imental error. A best-fit line helps average out these errors. These lines can be calculated with statistical formulas for regression lines; however, most spreadsheet applications, such as Micro- soft Excel®, are able to perform these calcula- tions. Best-Fit Line and Density The equation of a line is typically represented as y = mx + b, in which y represents a data point on the y-axis; x represents a data point on the x- axis; m represents the slope of the line, and b represents the y-intercept (i.e., where the line crosses the y-axis). When a graph of mass versus volume is created in this experi-ment, the y-intercept must be set to zero. This is because when there is no mass, there is no volume, and vice versa. The equation is then y = mx. Because the mass is graphed on the y-axis, and the volume is graphed on the x-axis, this equation actually shows that mass = (m)volume. Rearrangement of the equation to isolate m (the slope) results in the equation: mass/volume = m. Mass/volume is the mathematical formula for density. Thus, in a graph of mass versus volume, the slope (m) represents the density of the substance. EXPLORING DENSITY Materials Included in the materials kit: Needed from the equipment kit: Polyethylene cylinder Acrylic cylinder (clear) Graduated Electronic Pipets Spoons (white) cylinder balance 50-mL Aluminum cylinder (silver) Included in the materials kit: • Polyethylene cylinder (white) • Acrylic cylinder (clear) • Aluminum cylinder (silver) Weighing boats Plastic cups Wax pencil Ruler Needed from the equipment kit: • Graduated cylinder 50-mL • Electronic balance • Ruler • Pipets • Weighing boats • Spoons • Plastic cups • Wax pencil Needed but not supplied: • Sugar (sucrose, C12H22O12), 250 g • Water (bottled or purified), 1 L • Non-diet beverage containing natural sugar, 100 mL • Graphing program (such as Microsoft Excel®) Safety Safety goggles should be worn during this investigation. There are no addi- tional safety concerns. Read all of the instructions for this laboratory activity before beginning. Follow the instruc- tions closely. Observe established laboratory safety practices, including the use of appro- priate personal protective equipment (PPE) as described in the Safety and Procedure sections. Do not eat, drink, or chew gum during this activity. Wash your hands with soap and water before and after the activity. Clean the work area with soap and water after completing the inves- tigation. Keep pets and children away from lab materials and equipment. .............................................................................continued.................................................................................. [Show More]
Last updated: 1 year ago
Preview 1 out of 16 pages
Instant download

Buy this document to get the full access instantly
Instant Download Access after purchase
Add to cartInstant download
Reviews( 0 )
Document information
Connected school, study & course
About the document
Uploaded On
Aug 13, 2021
Number of pages
16
Written in
Additional information
This document has been written for:
Uploaded
Aug 13, 2021
Downloads
0
Views
60



.png)
.png)

.png)