GIZMO LEARNING: Student Exploration: Covalent Bonds. 100%.
Document Content and Description Below
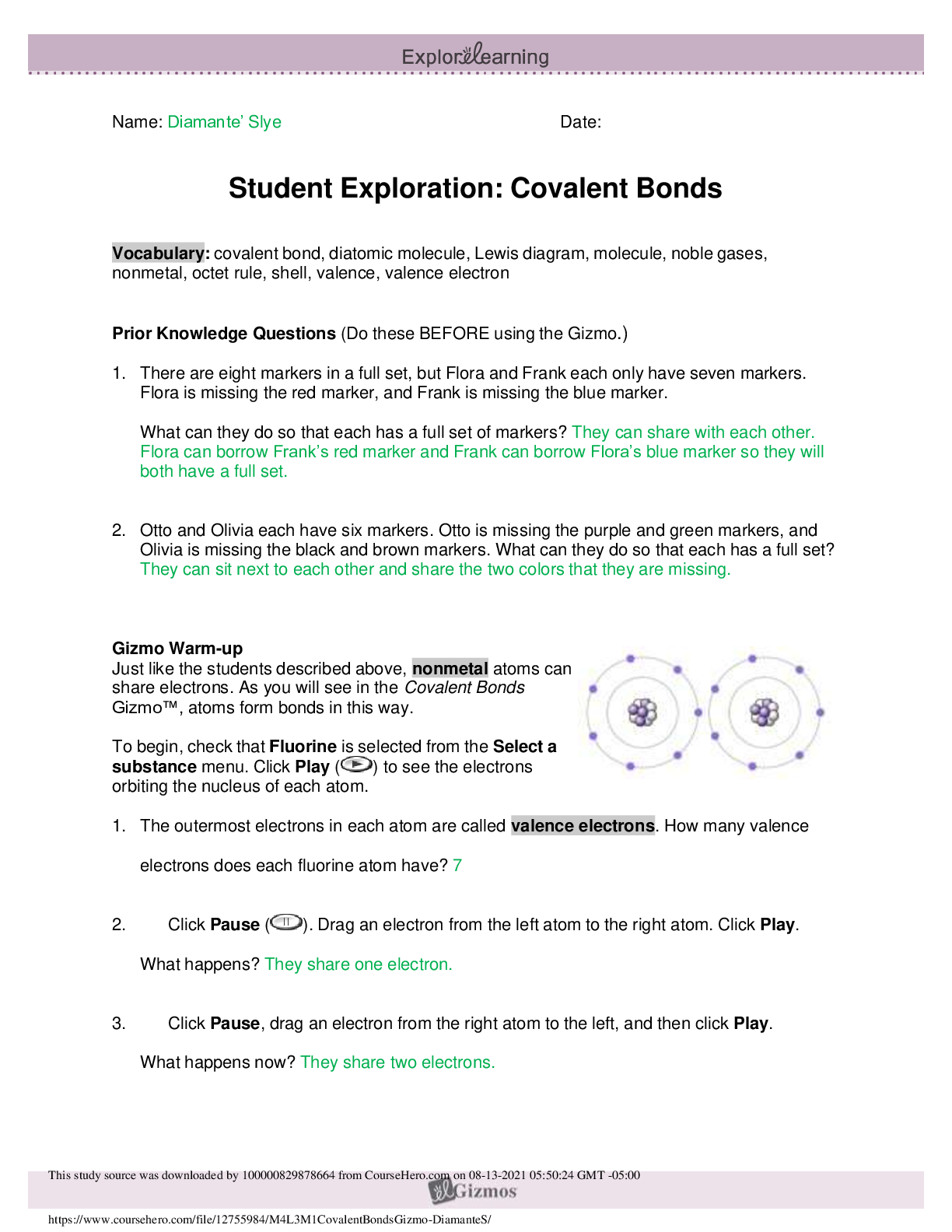
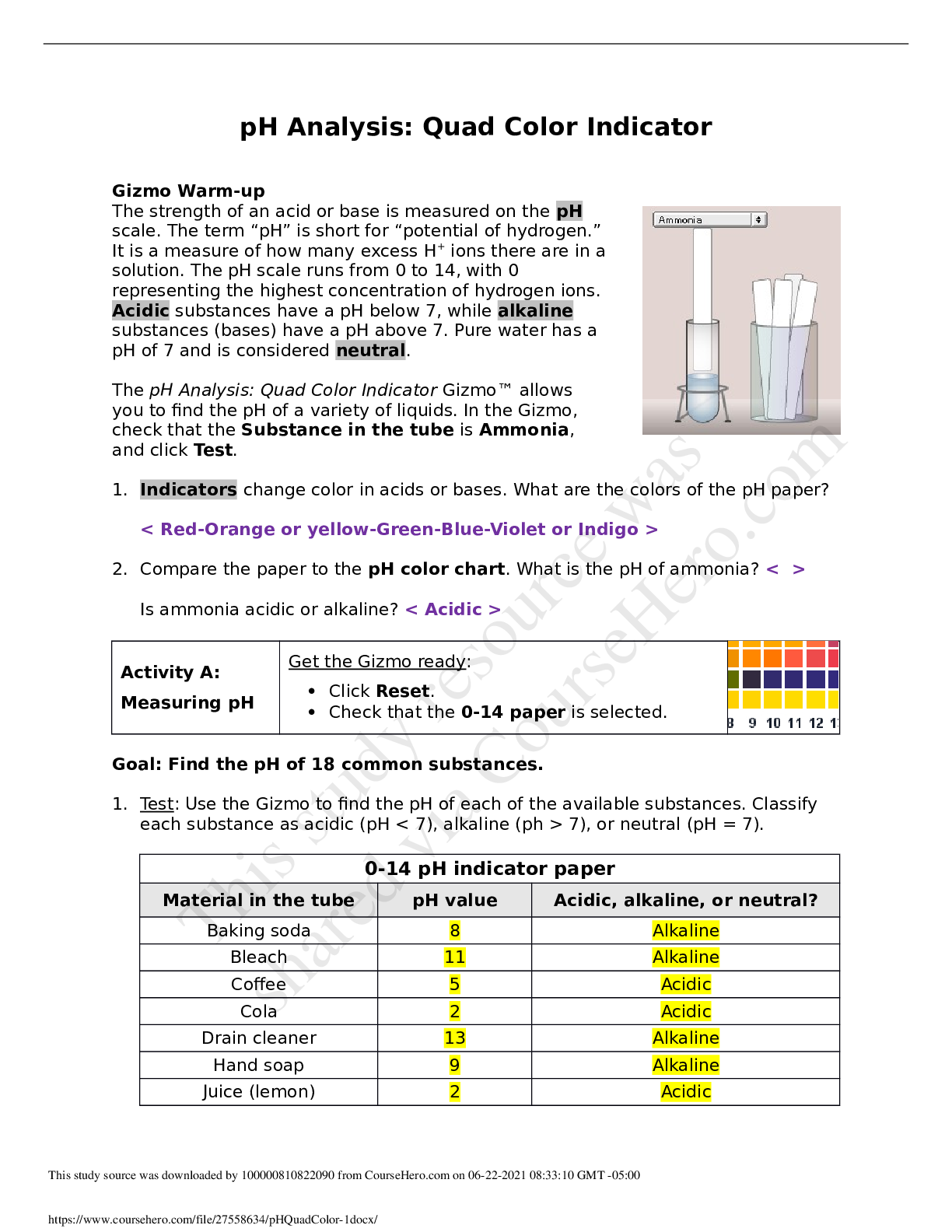
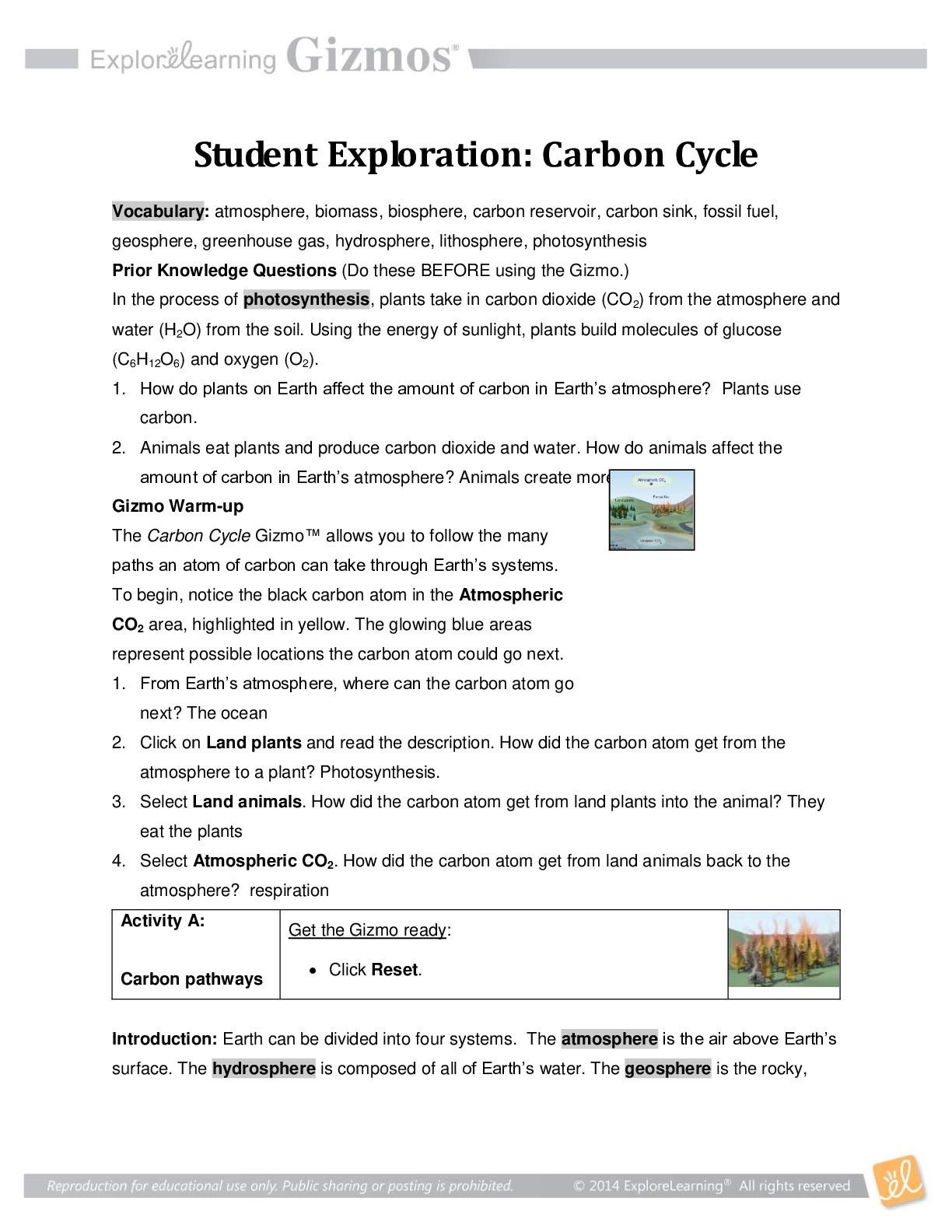
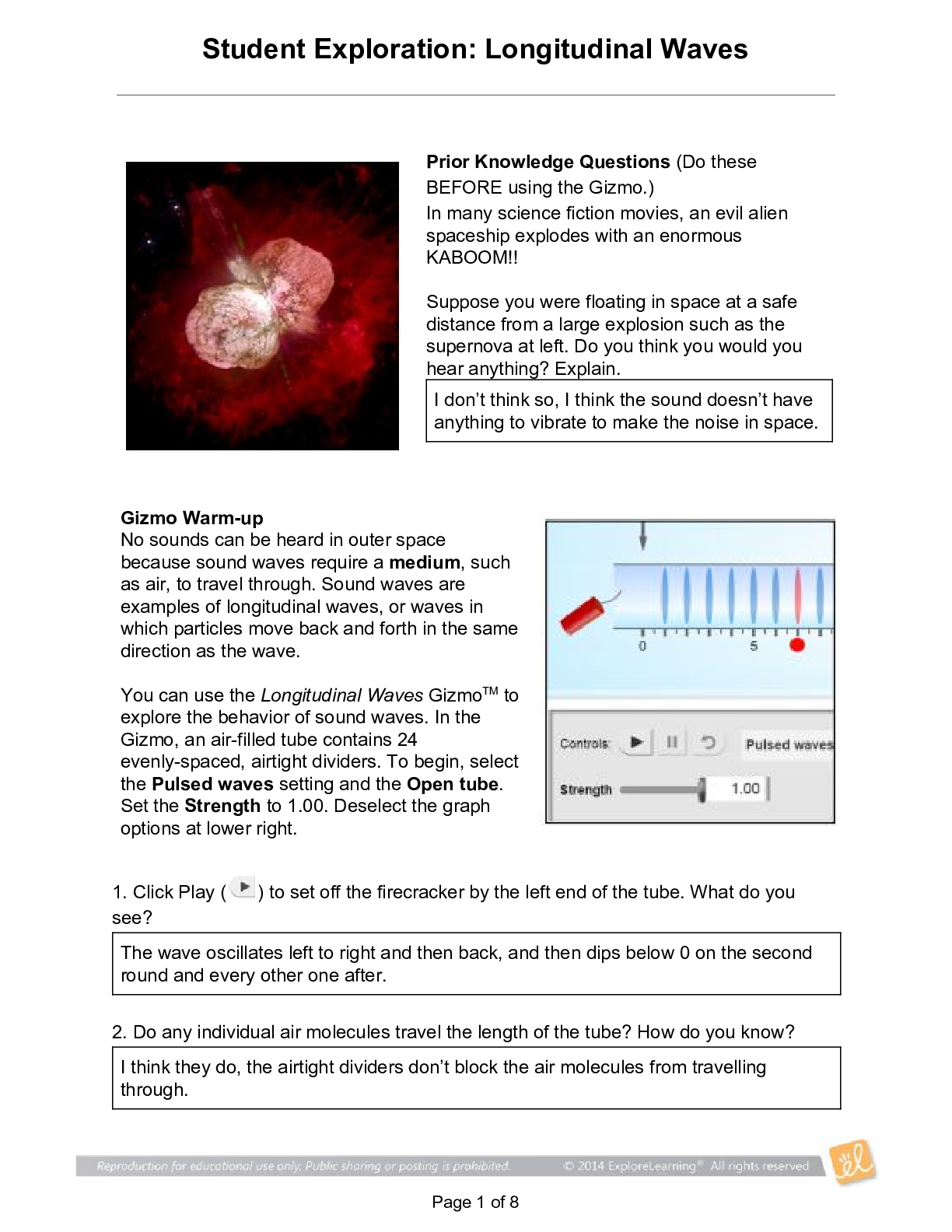
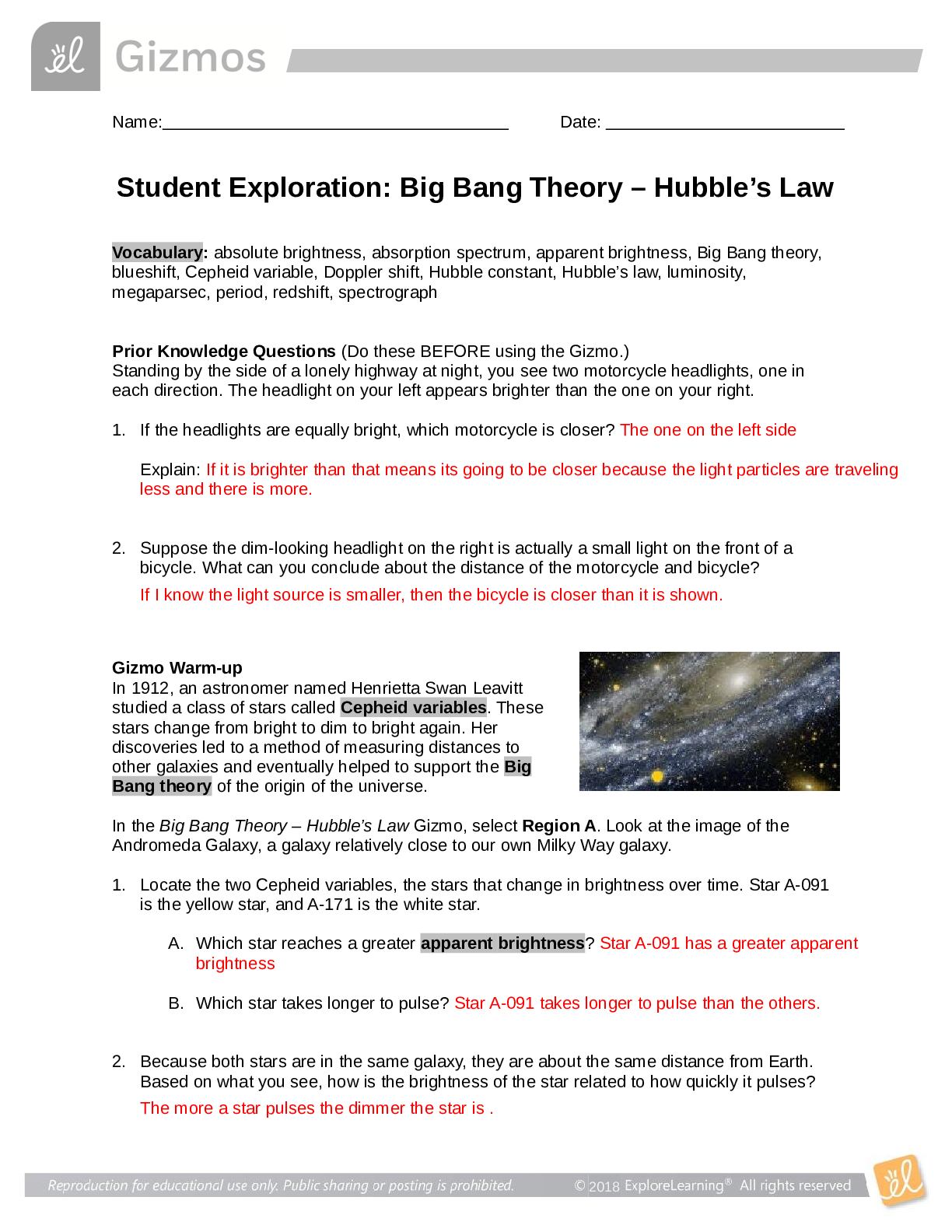
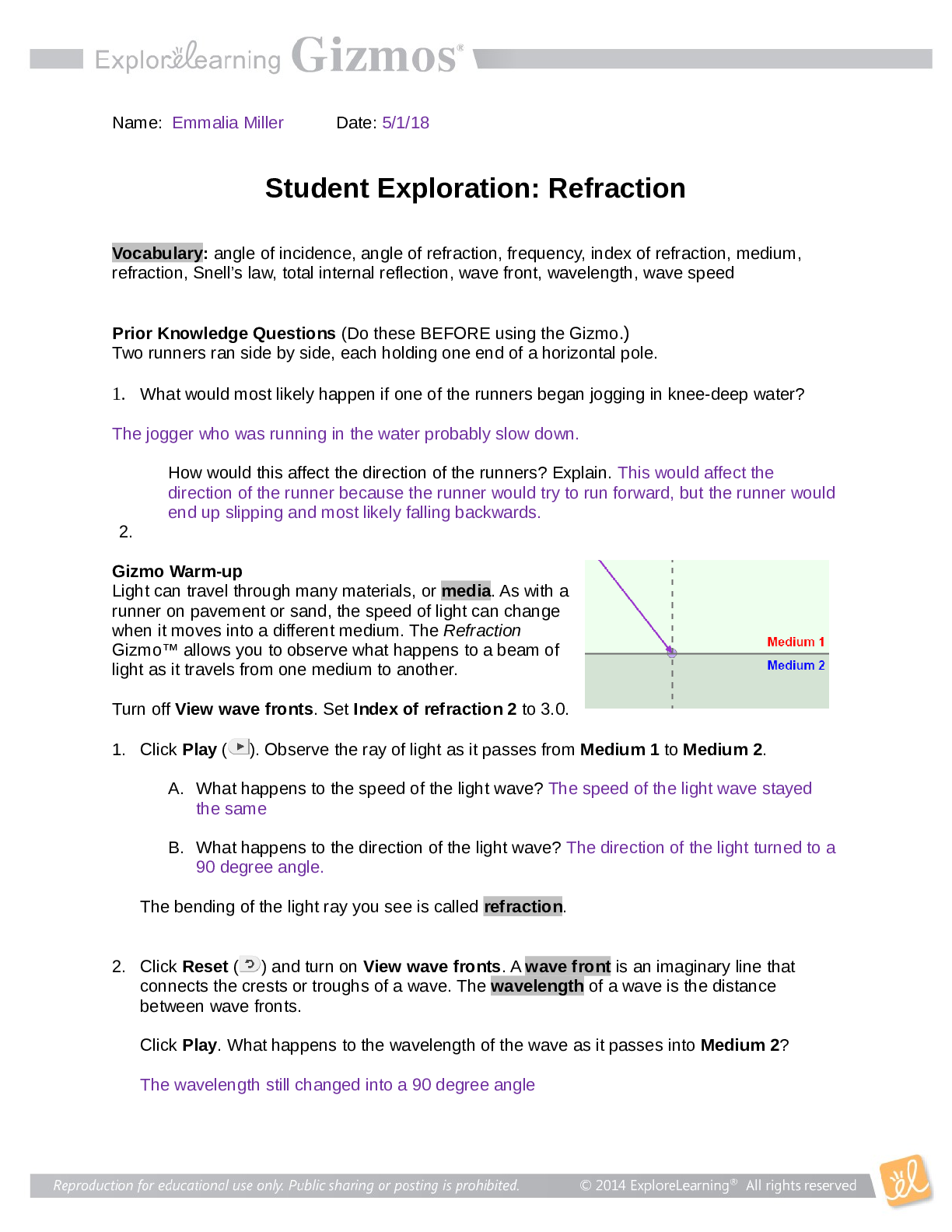
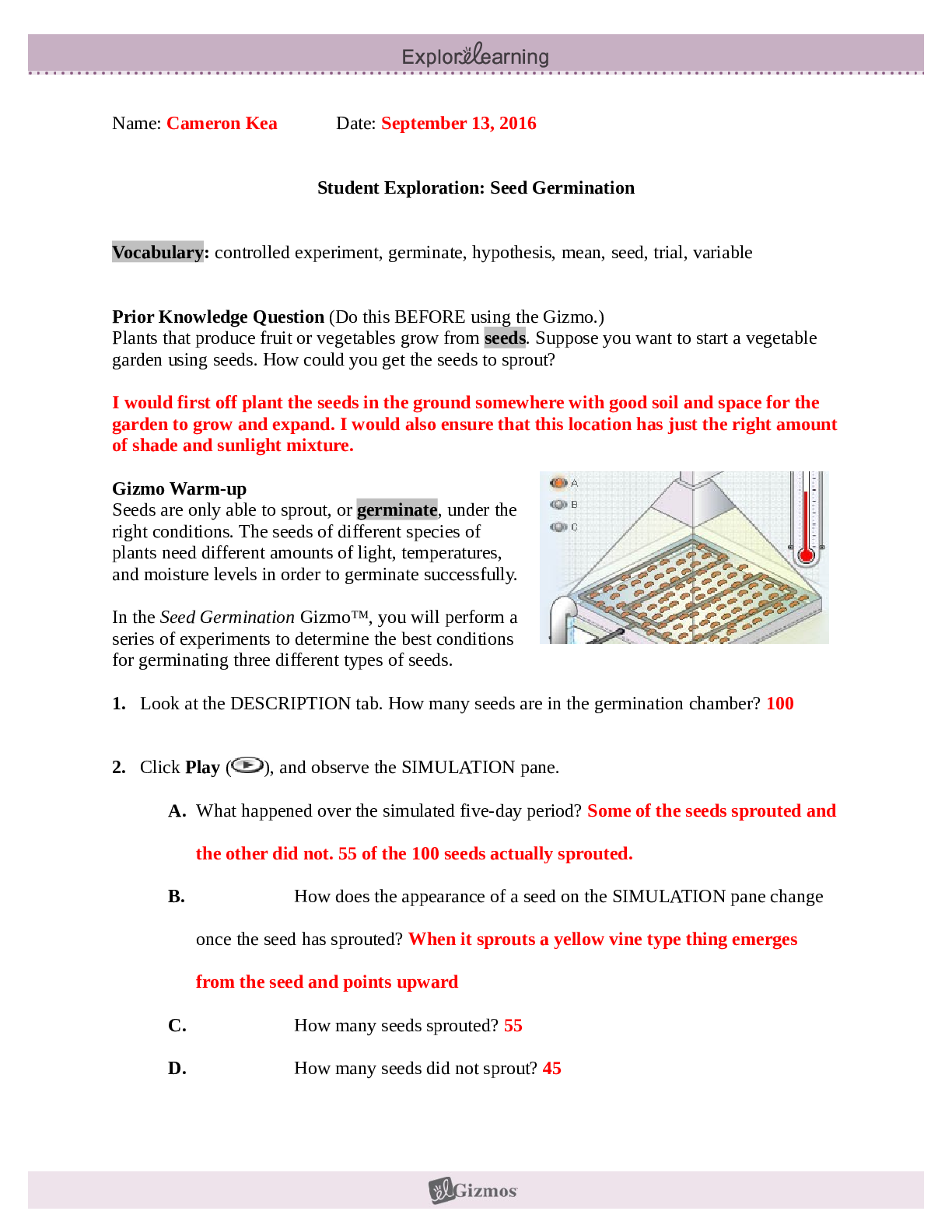
Student Exploration: Covalent Bonds CVPS Chemistry Fall 2014 Vocabulary: covalent bond, diatomic molecule, Lewis diagram, molecule, noble gases, nonmetal, octet rule, shell, valence, valence electro... n Prior Knowledge Questions (Do these BEFORE using the Gizmo.) There are eight markers in a full set, but Flora and Frank each only have seven markers. Flora is missing the red marker, and Frank is missing the blue marker. What can they do so that each has a full set of markers? Otto and Olivia each have six markers. Otto is missing the purple and green markers, and Olivia is missing the black and brown markers. What can they do so that each has a full set? Gizmo Warm-up Just like the students described above, nonmetal atoms can share electrons. As you will see in the Covalent Bonds Gizmo™, atoms form bonds in this way. To begin, check that Fluorine is selected from the Select a substance menu. Click Play ( ) to see the electrons orbiting the nucleus of each atom. The outermost electrons in each atom are called valence electrons. How many valence electrons does each fluorine atom have? Click Pause ( ). Drag an electron from the left atom to the right atom. Click Play. What happens? Click Pause, drag an electron from the right atom to the left, and then click Play. What happens now? Introduction: The electrons that orbit the nucleus of an atom are arranged into shells. The first shell contains up to two electrons and the second contains up to eight electrons. Most elements are stable when they have eight valence electrons—a rule of thumb known as the octet rule. (Elements with less than five electrons are stable with two valence electrons.) Question: What happens when atoms share electrons? Predict: Each hydrogen atom has one valence electron, but it needs two electrons to be stable. How can both hydrogen atoms each achieve a stable configuration? Form a bond: Drag the electrons so that they move around both hydrogen atoms. Click Play to observe them in orbit, and then click Check. You have created a covalent bond. Congratulations, you have completed a molecule of hydrogen! Because the molecule has two atoms, it is a diatomic molecule. Click the camera ( ) to take a snapshot of your completed molecule. Paste the image into a blank document and label the image “H2.” Draw a diagram: Covalent bonds are shown in Lewis diagrams. In a Lewis diagram, dots represent unshared valence electrons and dashes represent pairs of shared electrons. Turn on Show Lewis diagram. What is the Lewis diagram for hydrogen, H2? H H Form a bond: Now select Fluorine and create a molecule of fluorine, F2. Take a snapshot of this molecule and add it to your document. (Be sure to label each molecule you add.) What is the Lewis diagram for fluorine, F2? F F Think and discuss: How is the formation of covalent bonds similar to kids sharing markers? How is it different? If possible, discuss your answer with your classmates and teacher. Question: How do atoms share more than one pair of electrons? Observe: Like fluorine and most other elements, oxygen atoms are most stable with a full complement of eight valence electrons. How many valence electrons does each oxygen atom have now? How many more electrons does each oxygen atom need to be stable? Form a bond: Drag electrons back and forth until the molecule of oxygen (O2) is stable. Click Check to confirm your molecule is stable. Take a snapshot and paste the image into your document (don’t forget to label it “O2”). How many pairs of shared electrons are there in a stable molecule of oxygen? Draw a diagram: Draw a Lewis diagram of the oxygen molecule in the space below at left. To check your work, turn on Show Lewis diagram. Draw the correct diagram on the right. Practice: Create covalent bonds and stable molecules for the remaining substances. Take a snapshot of each completed molecule and add it to your document. Draw Lewis diagrams for each one. (As above, draw the diagram on your own before checking your work.) (Activity B continued on next page) Activity B (continued from previous page) Count: Review the Lewis diagrams you drew on the previous page. Note that each element tends to form a certain number of chemical bonds. This value is the valence of the element. For each element in the table below, use the Gizmo to find the number of valence electrons and the list the valence based on the Lewis diagram. Then find the sum of these numbers. Make a rule: If you knew the number of valence electrons in a nonmetal atom, how would you determine the valence of the element? (Hint: Ignore hydrogen for now.) Analyze: The first shell can hold a maximum of two electrons. How does this explain the valence of hydrogen? Apply: Selenium has six valence electrons. What is the valence of selenium? 2- Think and discuss: The last column of the periodic table contains the noble gases, elements that do not easily form chemical bonds.Why don’t these gases tend to form chemical bonds? [Show More]
Last updated: 1 year ago
Preview 1 out of 5 pages

Reviews( 0 )
Document information
Connected school, study & course
About the document
Uploaded On
Feb 12, 2021
Number of pages
5
Written in
Additional information
This document has been written for:
Uploaded
Feb 12, 2021
Downloads
0
Views
89

.png)








.png)





.png)

