GIZMOS. Student Exploration: Equilibrium and Pressure
Document Content and Description Below
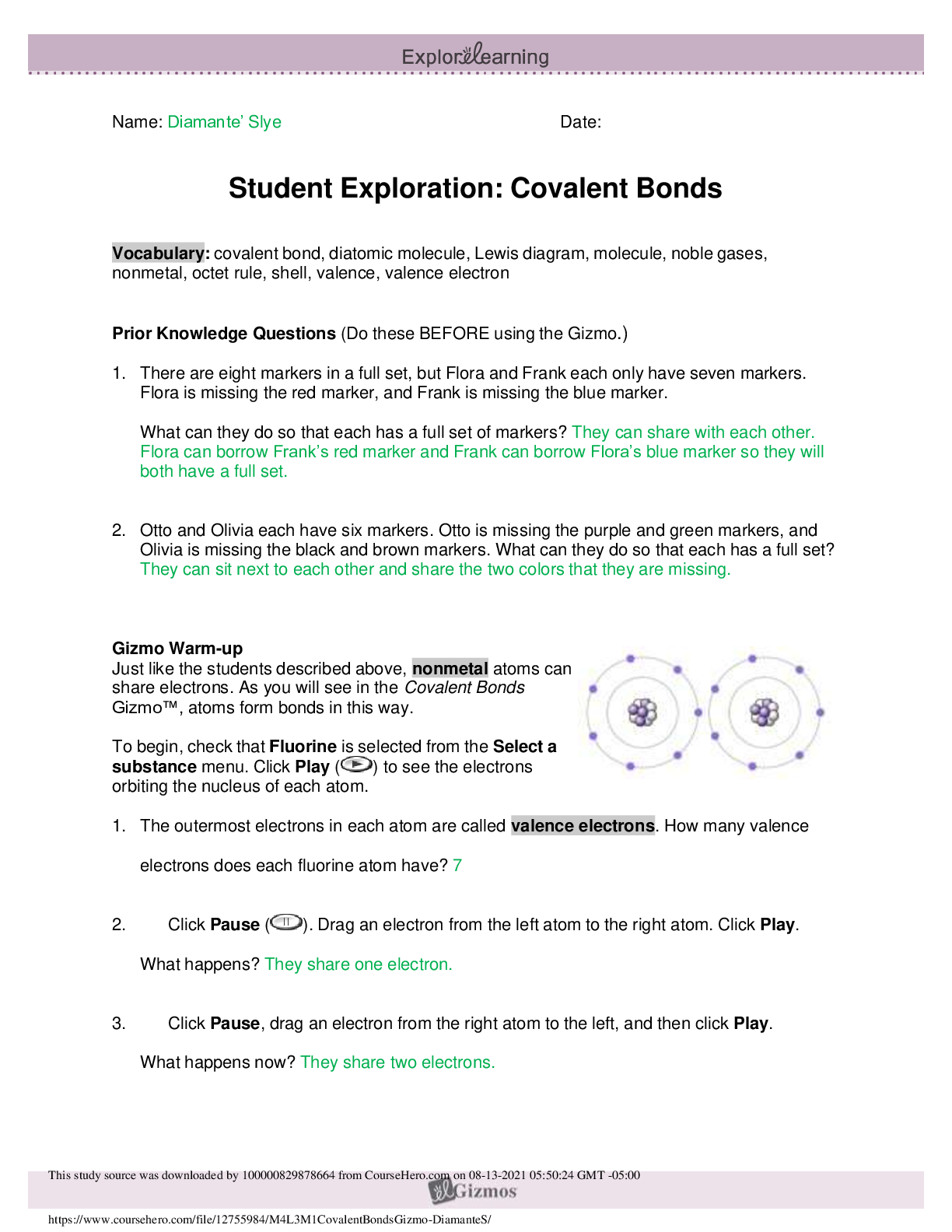
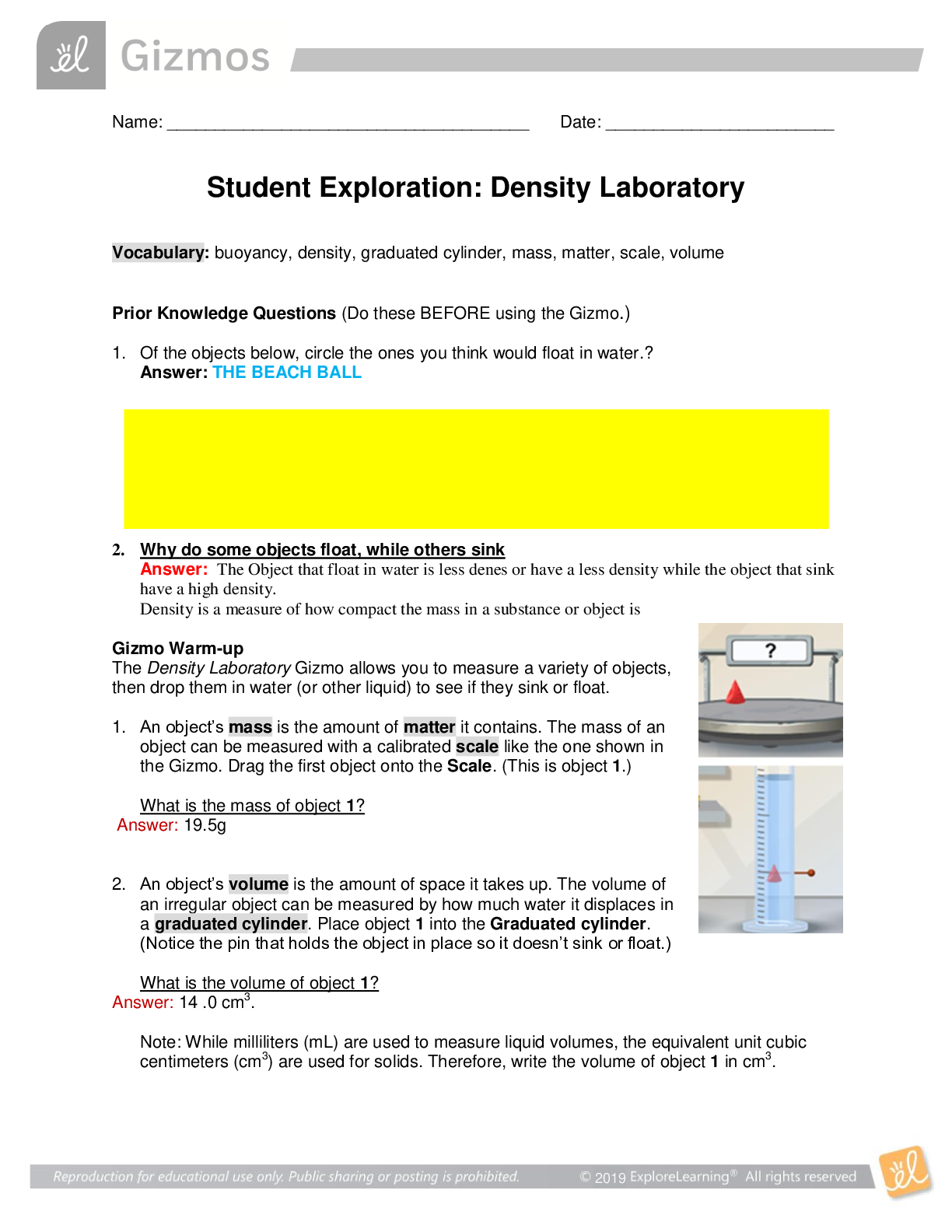
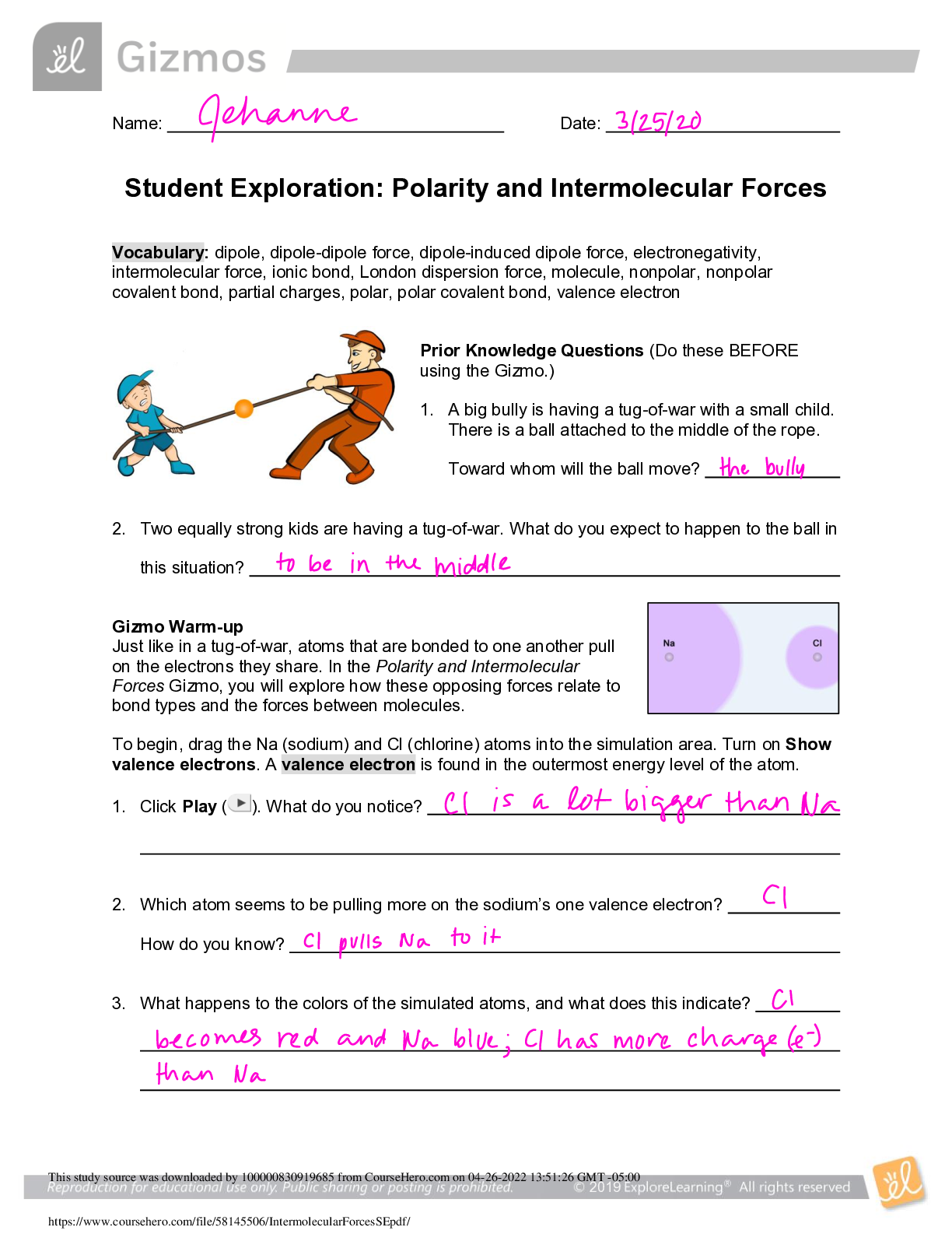
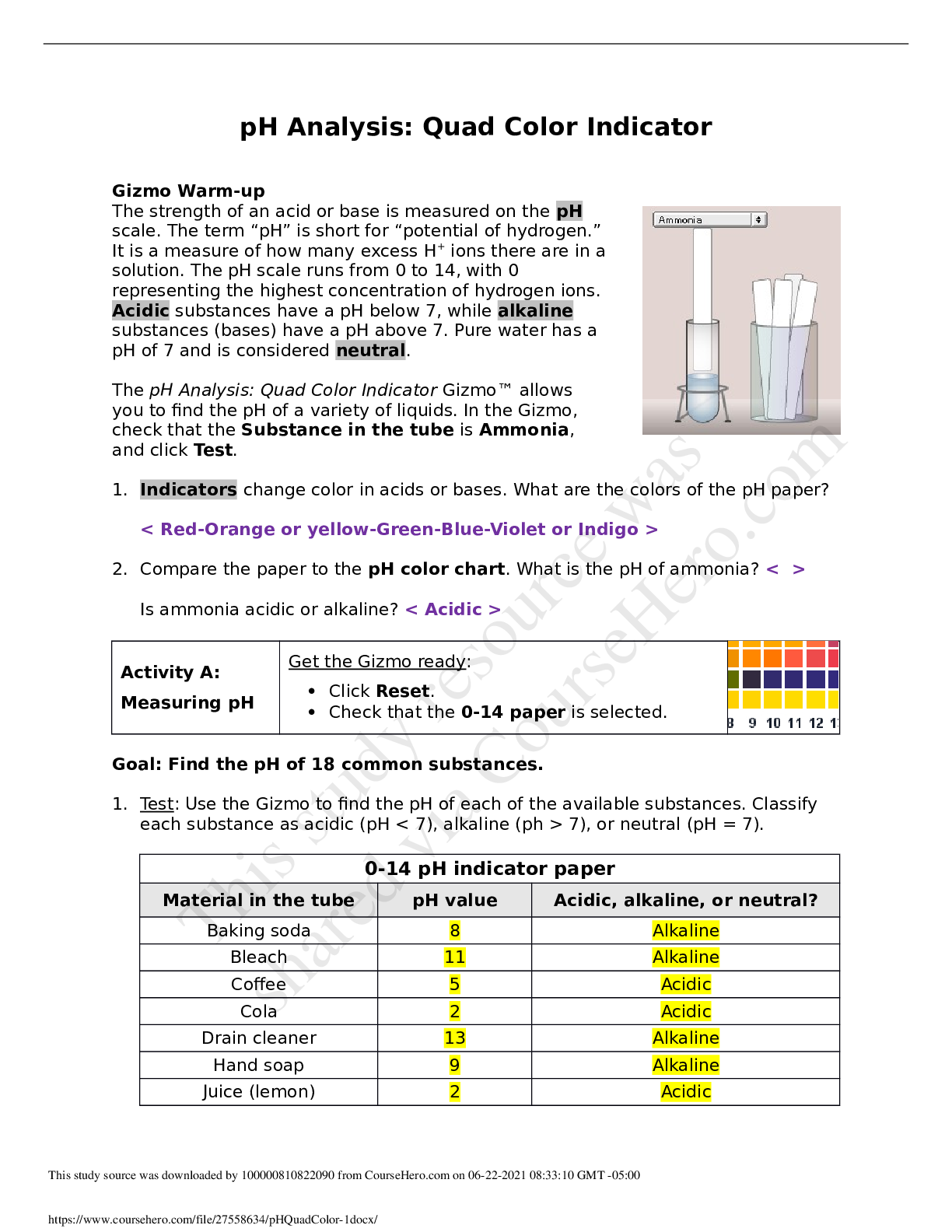
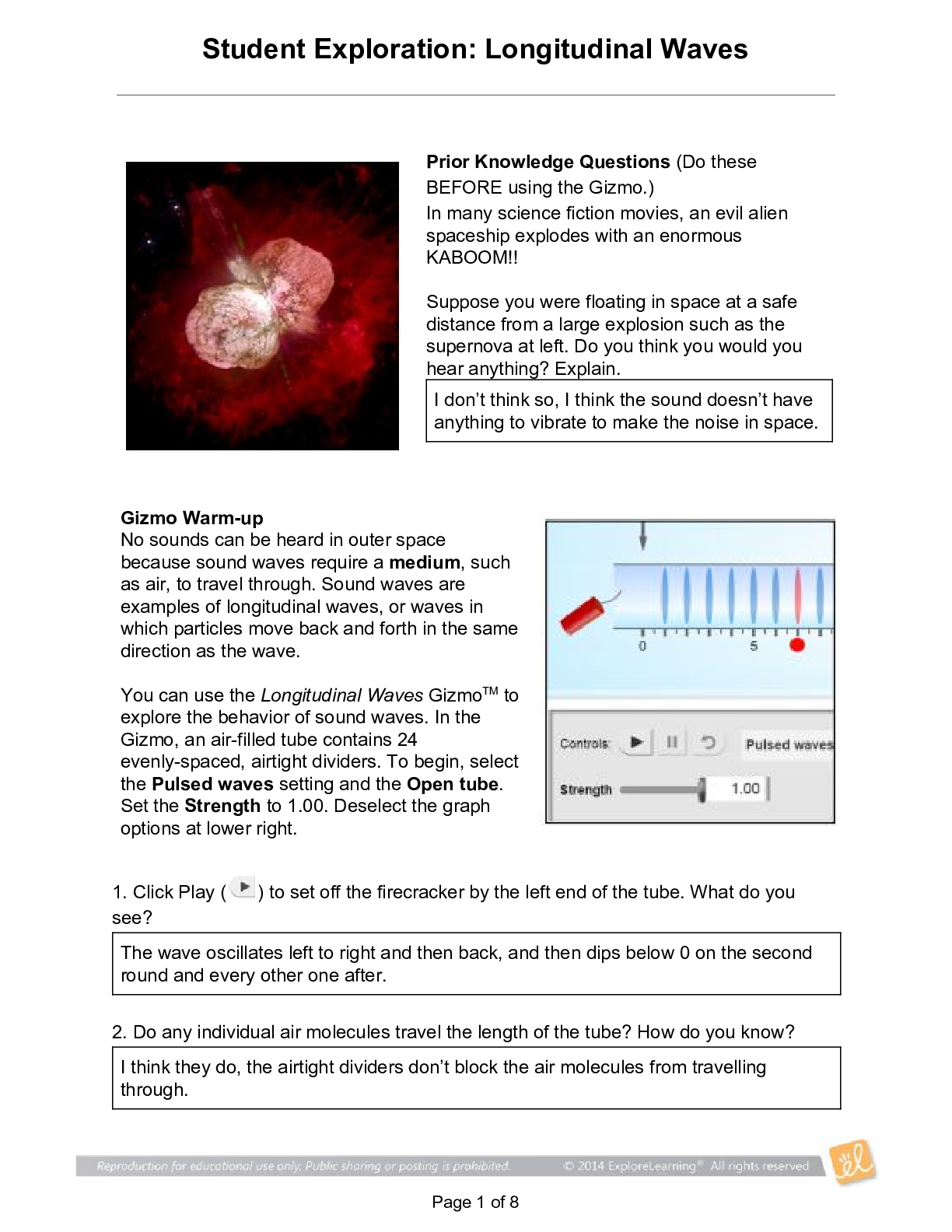
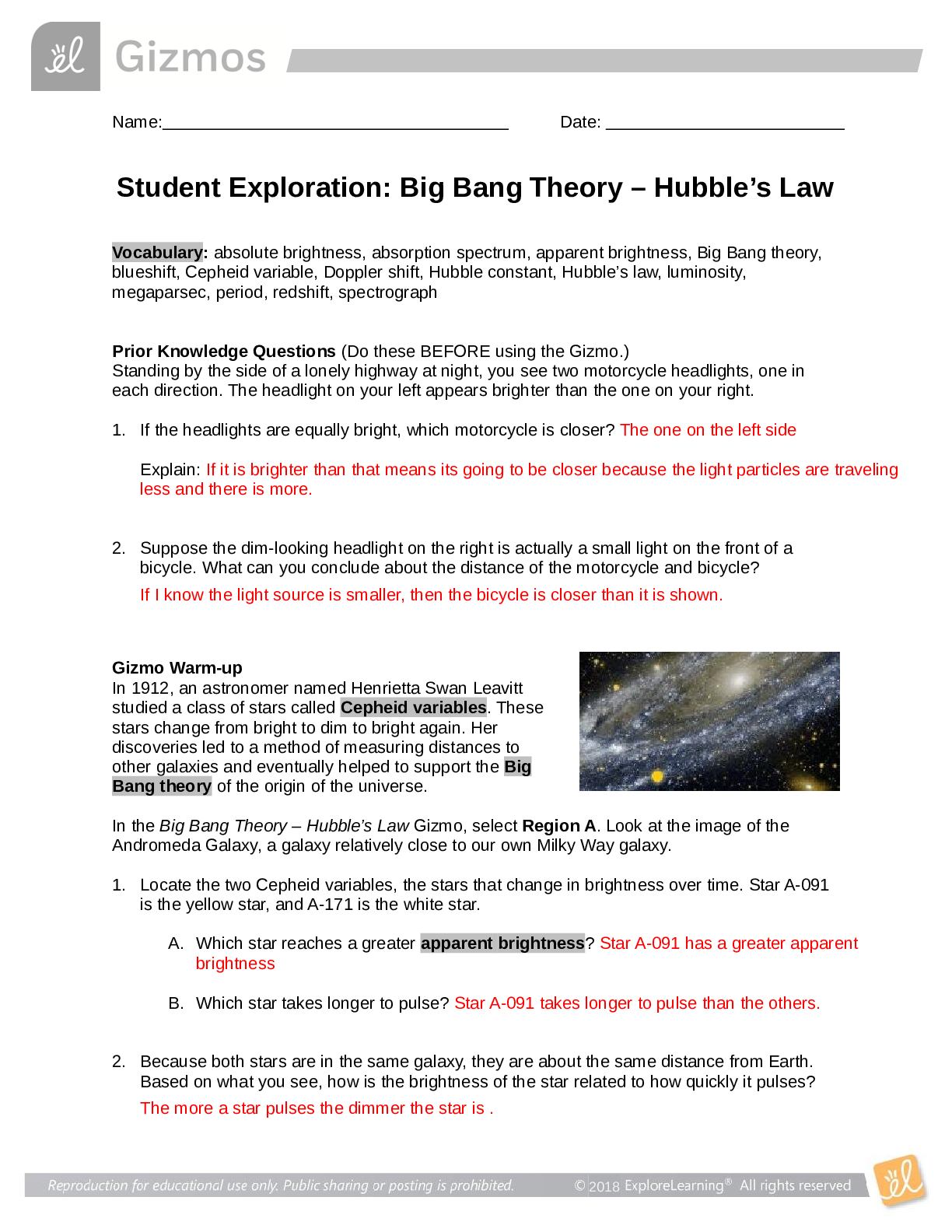
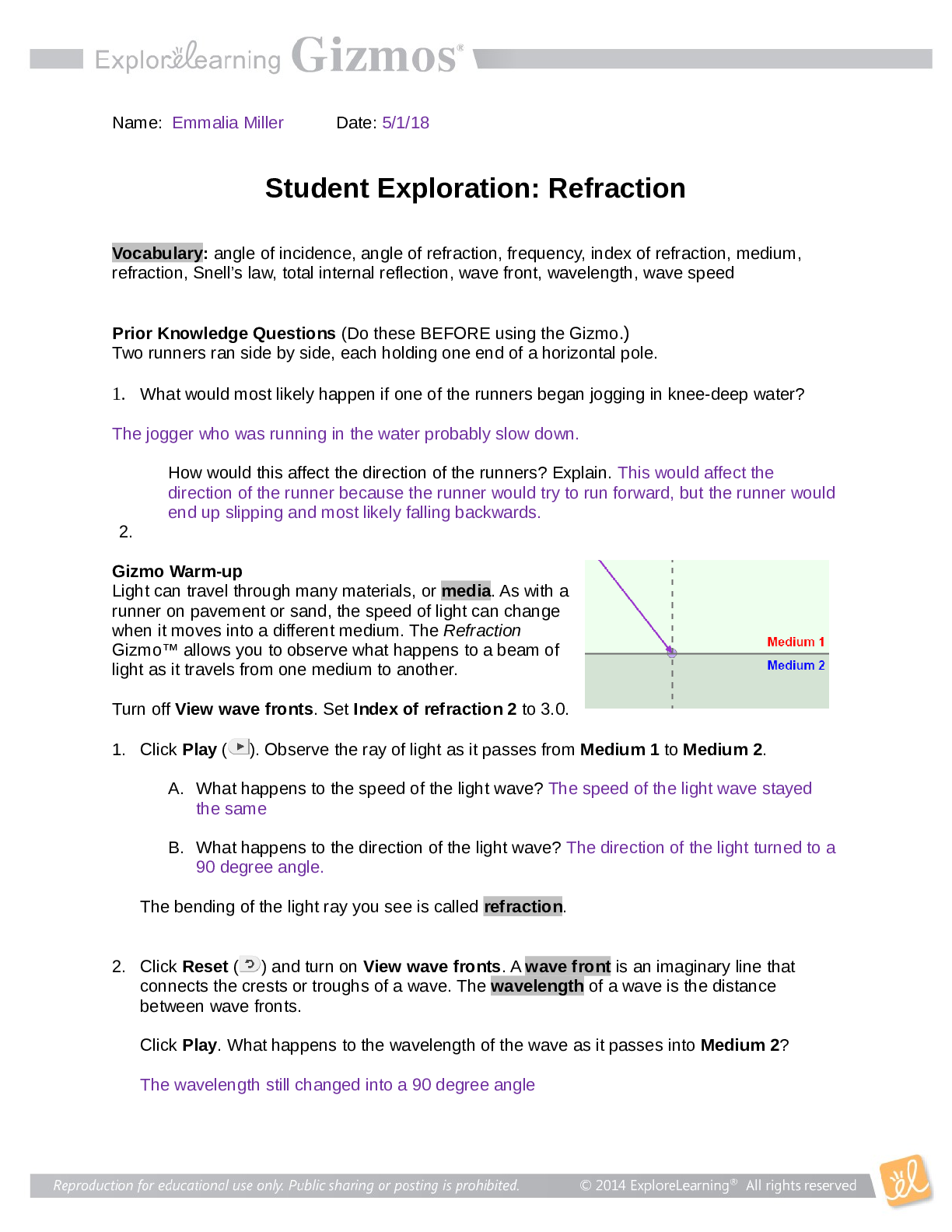
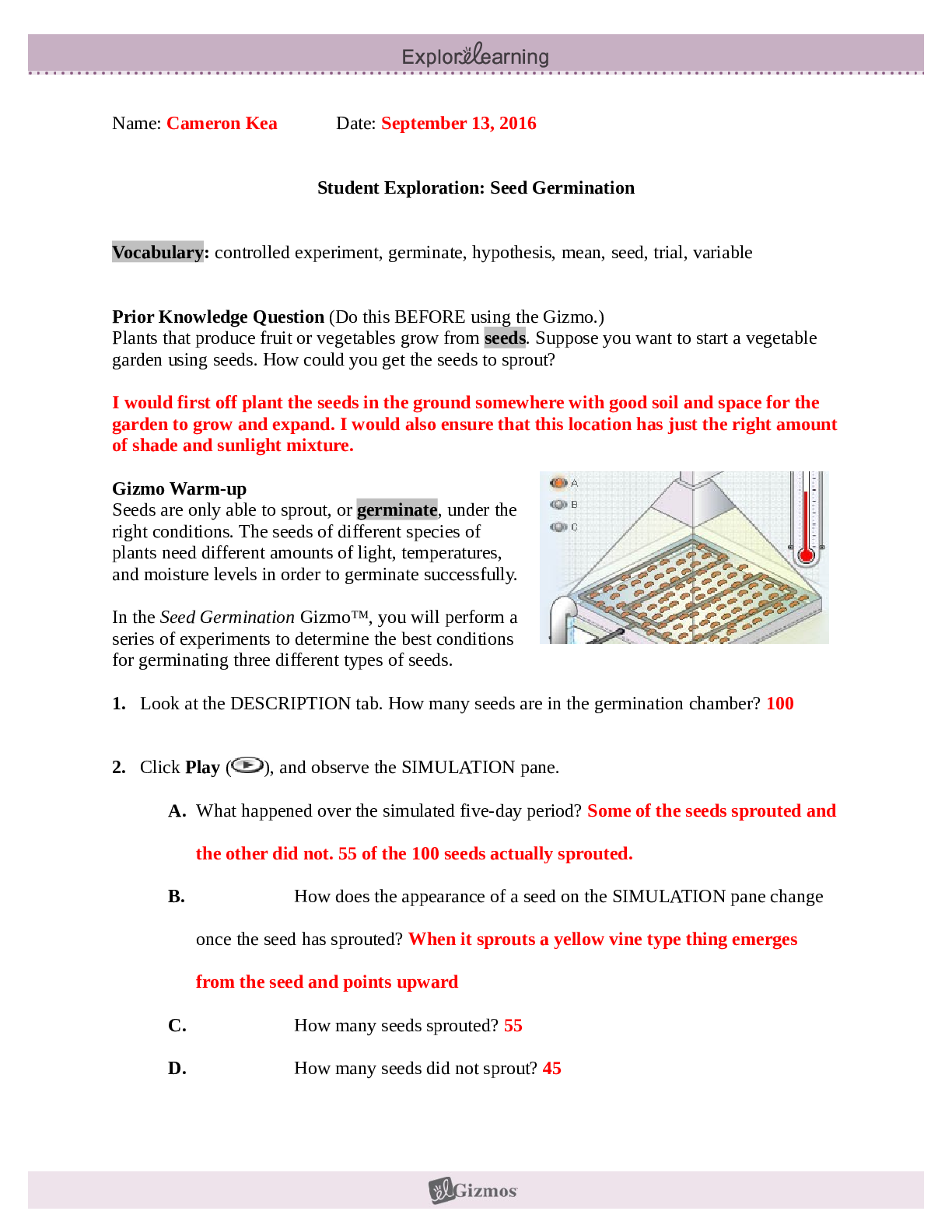
2019 Name: ______________________________________ Date: ________________________ Student Exploration: Equilibrium and Pressure [Note to teachers and students: This Gizmo was designed as a follow-up... to the Equilibrium and Concentration Gizmo. We recommend doing that activity before trying this one.] Vocabulary: Dalton’s law, Le Châtelier’s principle, partial pressure, pressure Prior Knowledge Questions (Do these BEFORE using the Gizmo.) A typical scuba tank has a volume of 11 liters and can support a diver for one hour. An adult breathes about 3 liters of air with each breath. 1. How can an 11-liter tank give a diver enough oxygen for one hour? 2. Why are diving cylinders made of thick, reinforced aluminum or steel? Gizmo Warm-up Gases consist of billions of tiny particles in constant motion, colliding with each other and the walls of the container. The sum of all these collisions creates pressure on the walls of the container. In theory, any amount of gas can be squeezed into a container if the container is strong enough to withstand the gas pressure. The Equilibrium and Pressure Gizmo shows a mixture of gases in a chamber. The lid of the chamber can move up or down. 1. Check that Reaction 1 is selected. Use the Moles NO2 slider to increase the number of NO2 molecules in the chamber. How does this affect the volume of the chamber? 2. Notice the weights on the lower right side of the Gizmo. Drag several of these weights to the lid. How does this affect the volume of the chamber? 2019 Activity A: Dalton’s law Get the Gizmo ready: • Remove all weights from the lid. • Set Moles NO2 and Moles N2O4 to 0. Introduction: In a mixture of gases, each gas contributes a partial pressure to the total pressure in the chamber. Because the chamber has a moveable piston, the pressure inside is equal to the pressure on the lid. In this Gizmo, the units of pressure are megapascals (MPa). Question: How do individual gases contribute to the total pressure in a chamber? 1. Observe: Set Moles NO2 to 2 to add 2 moles of NO2 gas to the chamber. A. The total pressure (P) on the chamber is shown next to the weights, at bottom right. What is the total pressure in the chamber B. Select the BAR CHART tab and select Pressure. Turn on Show data values. What is the pressure of NO2? PNO2 2. Observe: Set Moles N2O4 to 2 to add 2 moles of N2O4 gas to the chamber. A. What is the total pressure on the chamber B. What are the partial pressures of NO2 and N2O4? PNO2 = C. What is the sum of the partial pressures of NO2 and N2O4? Dalton’s law states that the total pressure in a container is equal to the sum of the partial pressures: P = P1 + P2 + …. + Pn 3. Explain: Why doesn’t the total pressure increase when more gas is added to the chamber? (Hint: What would you see if the volume of the chamber was fixed?) 4. Analyze: A molecule of N2O4 has twice the mass as a molecule of NO2. What do you notice about the partial pressure exerted by 2 moles of NO2 compared to the partial pressure exerted by 2 moles of N2O4? At a given temperature, the partial pressure exerted by a gas depends only on the quantity of the gas, not on its mass. Thus a mole of a light gas such as hydrogen (H2) exerts the same pressure as a mole of a heavier gas such as dinitrogen tetroxide (N2O4). 2019 Activity B: Partial pressure and equilibrium Get the Gizmo ready: • Select Reaction 2. • Move the Sim. speed slider all the way to the right. Introduction: In the Equilibrium and Concentration Gizmo, you found that reversible reactions eventually result in chemical equilibrium. Chemical equilibrium is reached when the rates of the forward and reverse reactions are the same. The constant Kc describes the ratio of products to reactants at equilibrium. A similar equilibrium can be calculated based on partial pressure. Question: How can partial pressure be used to measure equilibrium? 1. Review: What is the formula of Kc for reaction 2? Assume [NO] is the equilibrium concentration of NO, [NO2] is the equilibrium concentration of NO2, and [N2O3] is the equilibrium concentration of N2O3. 2. Gather data: Experiment with a variety of initial partial pressures of NO, NO2, and N2O3. For each set of initial partial pressures, use the Gizmo to determine the equilibrium partial pressures of each gas. Run three trials for each set of initial conditions. Use the data you collect to fill in the first four columns of the table. (Note that some NO2 molecules combine to form N2O4, so there may be less free NO2 than NO.) 3. Calculate: In the last column of the table, calculate the ratio of equilibrium partial pressures as shown. What do you notice about the values in the last column? 2019 Activity B (continued from previous page) 5. Analyze: The equilibrium constant can be expressed in terms of partial pressure (P) or concentration. The symbol Kp is used when partial pressure calculations are used. For the general chemical equation aA(g) + bB(g) ⇌ cC(g) + dD(g) we have: (Note: The symbols a, b, c and d represent coefficients for substances A, B, C, and D.) How are the equations for Kp and Kc similar? 6. Explore: Select Reaction 3. In this reaction, a solid (C) reacts with a gas (CO2) to produce another gas (CO). To see how the solid affects the equilibrium, record the equilibrium partial pressures of CO2 and CO for each initial amount of C. Leave the last column blank for now. 7. Analyze: If possible, compare your results to those of your classmates. Based on your results, how does the initial amount of carbon (C) affect the equilibrium partial pressures of the two gases? Are there any consistent trends in the data? 8. Calculate: Because solids do not contribute to the pressure in the chamber, the amount of solid does not affect the equilibrium partial pressures of the gases. To calculate K in this reaction, ignore the solid: Use this equation to calculate Kp for each trial and fill in the last column of the table. 9. Think and discuss: On a separate sheet, explain why the amount of solid does not affect the equilibrium as long as there is enough solid to cover the bottom of the container. 2019 Activity C: Le Châtelier’s principle Get the Gizmo ready: • Click Reset. Select Reaction 2. • Set Moles NO, Moles NO2, and Moles N2O3 to 5. Introduction: In the Equilibrium and Concentration Gizmo, you learned that you can predict the direction of a reaction by comparing the reaction quotient (Qc) with the known equilibrium constant Kc. You can do the same thing using partial pressures: Question: How do changes in pressure affect the reaction equilibrium? 1. List: Click Play ( ). Select the BAR CHART tab, allow the reaction to proceed to equilibrium, and then click Pause ( ). What are the equilibrium partial pressures? 3. Predict: If you add weight to the lid, it increases the pressure on the chamber. A. How do you expect increasing pressure on the chamber to affect the partial pressures of the gases B. How do you expect the increased pressure to affect the equilibrium? 4. Calculate: Add all five weights to the lid. A. What is the partial pressure of each substance now? PNO 32 PNO2 32 PN2O3 32 B. Calculate Qp for this setup. C. In the current situation, is there an excess or products or reactants? Explain: (Activity C continued on next page) Activity C (continued from previous page) 5. Test: Click Play. Allow the reaction run until it reaches equilibrium again. A. What is the partial pressure of each substance now? B. Calculate Kp for this setup. C. How does this value of Kp compare to the value you found before? D. As the experiment reached equilibrium again, did the reactants or products increase? As the pressure on the system increased, the reaction shifted toward the side with fewer molecules—in this case the products. You may have noticed the volume of the chamber decreased slightly as the reaction proceeded. This is an example of Le Châtelier’s principle, which is the tendency of a system in equilibrium to shift in response to a change. 6. Apply: Select Reaction 5. If you were making water (H2O) from oxygen (O2) and hydrogen (H2), would it be an advantage to increase the pressure? Explain your answer, and then use the Gizmo to check your work. 7. Think and discuss: Why do you think increasing the pressure has the effect of shifting the equilibrium toward the side with fewer molecules? If possible, discuss your answer with your classmates and teacher. [Show More]
Last updated: 1 year ago
Preview 1 out of 9 pages

Reviews( 0 )
Document information
Connected school, study & course
About the document
Uploaded On
Jun 17, 2021
Number of pages
9
Written in
Additional information
This document has been written for:
Uploaded
Jun 17, 2021
Downloads
0
Views
135

.png)