*NURSING > STUDY GUIDE > NR 507 Week 4 Midterm Exam (Study Guide) | Download To Score An A (All)
NR 507 Week 4 Midterm Exam (Study Guide) | Download To Score An A
Document Content and Description Below
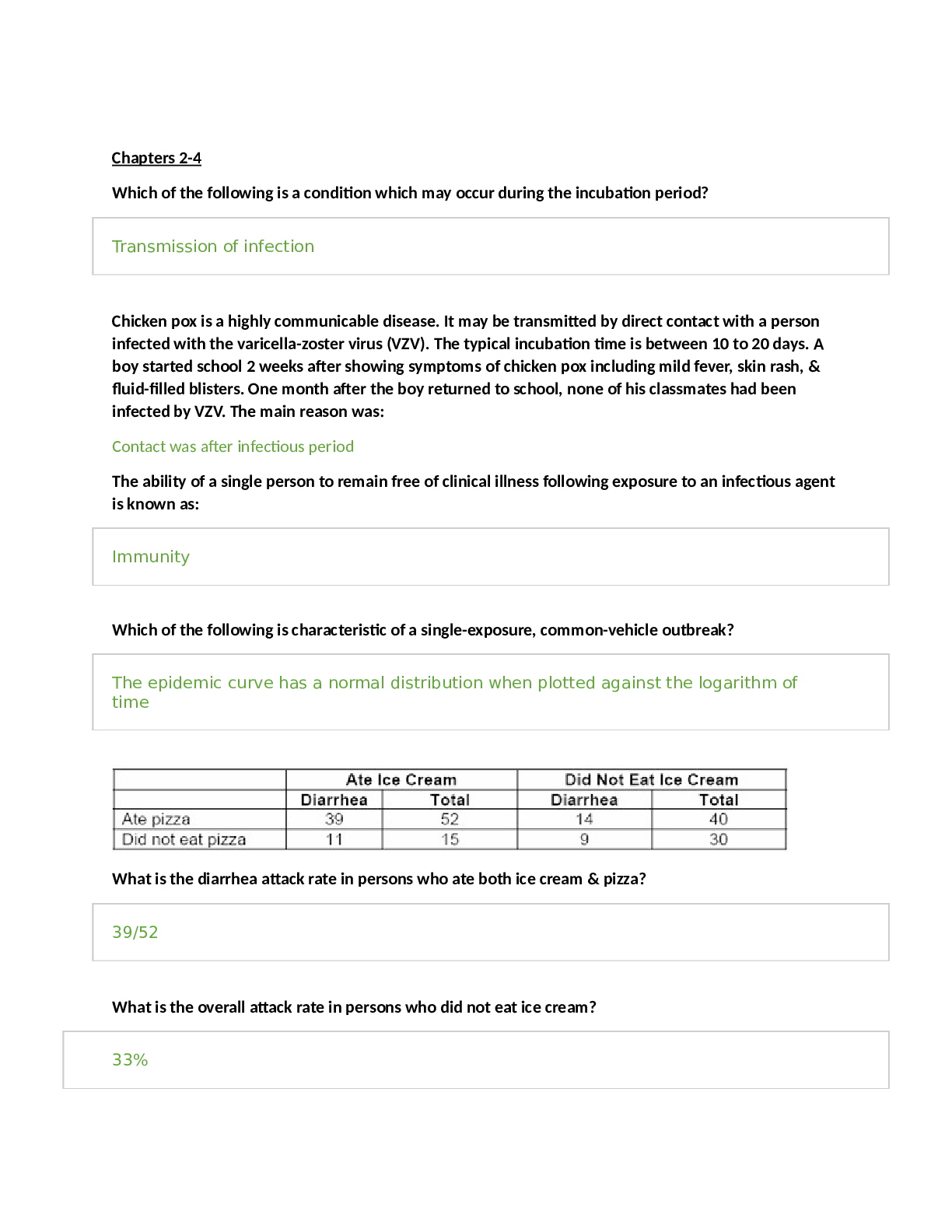
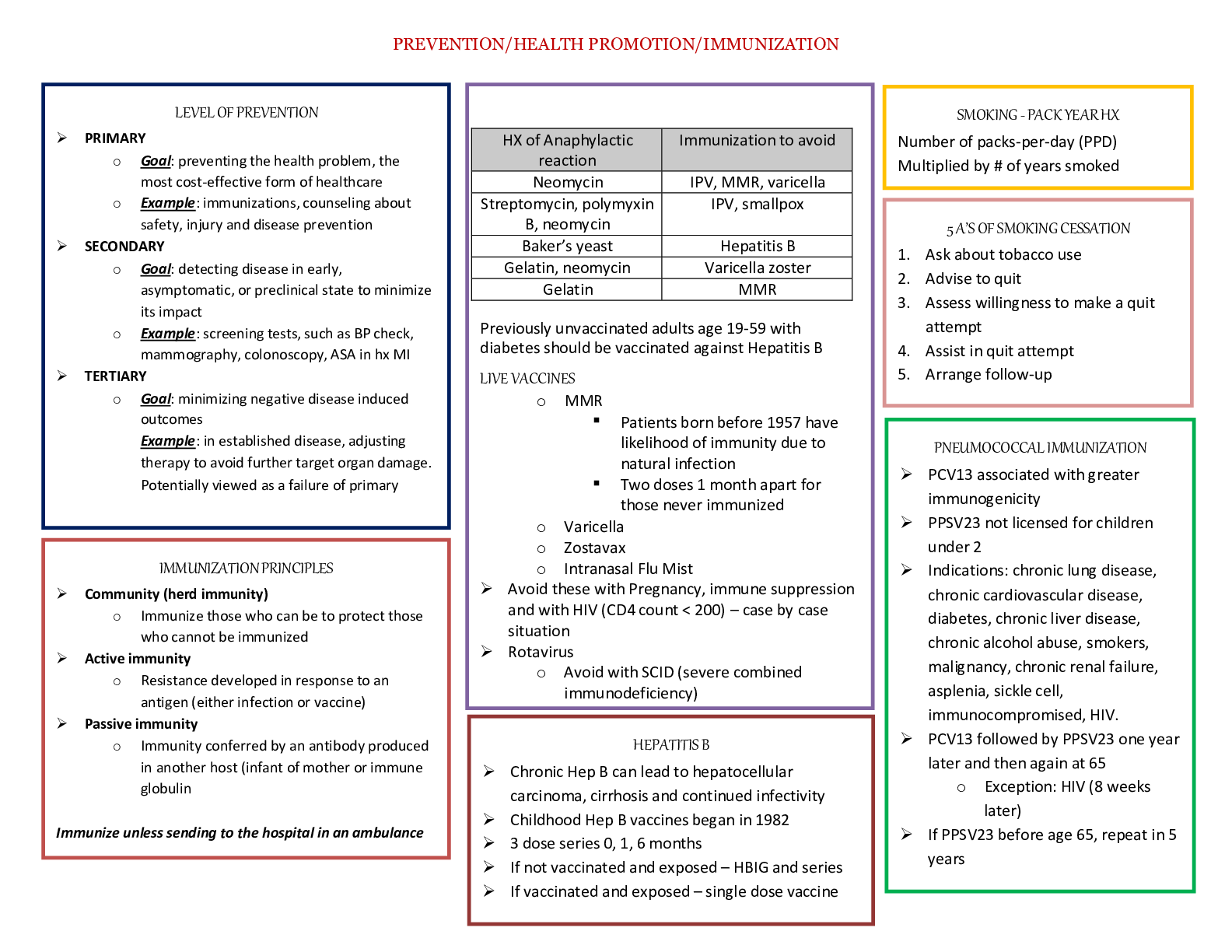
Mid Term Study Guide NR 507 Pulmonary 1. Concepts of anticholinergic drug and asthma: Anticholinergic drugs: block acetylcholine binding (primarily in the lungs) -promotes bronchiole dilation throu... gh decrease in the parasympathetic response (tiotropium & ipratropium) is fast acting 2. Bronchitis & associated pathogenesis: Begins with an exposure to an irritant (tobacco smoke) – activates bronchiole smooth muscle constriction- mucus secretion- release of inflammatory mediators(histamine, prostaglandins & leukotrienes) normal response to occasional airborne site irritants- over bronchitis is over long term 3 months for over 2 consecutive years- = smooth muscle hypertrophy = increase bronchoconstriction, hypertrophy and hyperplasia of goblet cells= mucus hypersecretion, epithelia cell metaplasia = non-ciliated squamous cells, migration of more WBCs to site = inflammation and fibrosis in bronchial wall, thickening and rigidity of bronchial basement membrane= narrowing of bronchial passageways : Increased mucus production- inflammation process = weight loss, loss of appetite, muscle weakness (interleukin controls appetite) increases protease activity= breakdown of elastin in the connective tissues of the lung= destruction of the wall between the alveoli and lungs = large ineffective air sacs develop-elastic recoil of bronchial wall -destroys bronchi and cant dilate and they stay constricted =air trapping : Chronic bronchitis =Dyspnea- air trapping increased mucus, increase WOB r/t chronic bronchoconstriction Cough- irritated and inflamed bronchial epithelia membrane Hypoxia & Hypercapnia -from impaired gas exchange 3. Chronic bronchitis and related acid/base disturbance: Hypercapnia (CO2 retention) = Respiratory acidosis r/t anatomical changes ventilation is compromised esp. exhalation = alveolar hyperinflation) expanded thorax) hypercapnia CO2 retention = respiratory acidosis\ 4. Perfusion: the actual exchange of O2 and CO2 in the bloodstream occurs via the alveoli and pulmonary capillaries: the passage of fluid to an organ or a tissue usually referring to delivery of blood to an area 5. Blood flow between the heart and lungs in chronic bronchitis: Poor ventilation leads to r to l shunting to occur= deoxygenated blood passes from r ventricle to the lungs to the l ventricle without adequate perfusion (gas exchange) the kidneys respond by secreting erythropoietin increasing RBC production the increase in RBC increase O2 carrying capacity -the increase blood volume increases the workload of the pulmonary and cardiovascular systems increasing blood volume and vasoconstriction = pulmonary HTN= increase workload on the R ventricle =cardiac hypertrophy= R side HF or Cor Pulmonale 6. Asthma signs and symptoms: Coughing esp. at night, chest tightness, shortness of breath, wheezing on exhalation, and rapid breathing: Characteristics: airway inflammation, bronchial hyperactivity, smooth muscle spasms, = excessive mucus production, hypertrophy of bronchial smooth muscle -obstruction and decrease alveolar ventilation 7. Bronchioles in asthma: There are 3 layers of the bronchiole which is a tube-like structure surrounding the lumen or airway passageway: innermost layer is composed of columnar epithelial cells and goblet cells- The outermost layer is composed of smooth muscle cells responsible for the ability of the airway to constrict and dilate – the middle layer is the laminar propria and it is embedded with connective tissue and immune cells: in asthma these protective features go overactive =inflammation response= damage to host tissue=hypertrophy of the bronchioles smooth muscle and excessive mucus production: bronchioles spasm-mucus production-obstruction 8. Alveolar hyperinflation with asthma: Increase mucus production from the goblet cells in the inflammation process forms plugs of mucus and pus and block alveolar passageways leading to air trapping and hyperinflation = erosion of airway tissue 9. Polycythemia Vera: a rare blood disease in which the body makes too many RBCs making the blood thicker than normal causing blood clots; is often a result of chronic low levels of O2 in the blood, the kidney compensates by increase secretion of erythropoetin, the primary hormone responsible for stimulating RBC production= as a result patients with chronic bronchitis will often exhibit increased HCT levels and can develop a condition called secondary polycythemia vera. 10. Mechanism of action of anticholinergic drugs to treat asthma: Anticholinergic drugs bind to muscarine receptors and block the action of acetylcholine They reduce Broncho motor tone =bronchodilation: block acetylcholine binding, bronchodilation, decrease parasympathetic response Cardiovascular: 11. Review concepts of Cardiac Output: Cardiac Output= Heart rate x Stroke volume\ Cardiac output: is the volume of blood ejected by each ventricle per minute (75 bpm x 70ml = 5.25 L/min) 5 L of blood in the body= every drop of blood circulates the body -per heartbeat per minute Cardiac output decrease with age at a rate of 1% per year after the age of 30 (other factors can accelerate the rate of decline) Cardiac output is a key component of HF and is important to understand the connection b/t HR & SV: DECRESE in HR (longer filling time) INCREASE in SV INCREASE in HR (shorter fill time) DECREASE in SV 12. Concepts of cardiac contractility: Contractility (ionotropic state) Contractility is determined by Calcium availability and its interaction with actin and myosin Contractility INCREASES by sympathetic stimulation (fever, anxiety, increased thyroxine levels; factors that increase the cellular metabolic rate will increase strength of muscle cell at least temporarily) Contractility DECREASES by low levels of ATP (ischemia, hypoxia, acidosis; Factors that decrease the amount of energy available to the muscle cell will decrease strength of contraction) 13. Preload and Afterload Concepts: PRELOAD: is the degree of myocardial fiber length stretch before contraction; the degree of stretch will be influenced by the end diastolic ventricular volume (EDV) [edv= is the amount of blood entering each ventricle during diastoles {approx. 120- 130 ml}] PRELOAD IS LOADING THE HEART WITH BLOOD Preload can be INCREASED by CHF, hypervolemia, (increased BV) Preload can be DECREASED by cardiac tamponade, hypovolemia (hemorrhage or dehydration) AFTERLOAD is the amount of tension the ventricle must develop during systole to open the semilunar valves and eject blood into circulation: Afterload is influenced by: Ventricular wall thickness= muscle strength Arterial pressure= resistance to ejection Ventricle chamber size= blood volume capacity Ventricular wall thickness: muscle strength (increase thickness the more muscle mass decrease tension force required for ejection) Arterial pressure: resistance to ejection (increase pressure within the pulmonary or systemic vessels = increase tension required for ejection) Ventricle chamber size: increased blood volume (increase tension required for ejection) AFTERLOAD INCREASED by systemic HTN, valve disease, COPD = pulmonary HTN AFTERLOAD DECREASED by hypotension or vasodilation, such as SHOCK LAPELACE LAW: THE HEART MUST WORK HARDER (INCREASE TENSION) WHEN HEART MUSCLE IS WEAK: VENTRICLES ARE HYPERTROPHIED (HOLD MORE VOLUME) AND OR PULMONARY OR SYSTEMIC BP IS ELEVATED 14. Systole and Diastole: Systole and Diastole are two phases of the cardiac cycle: they occur as the heart beats, pumping blood through the body. Systole: occurs when the heart contracts to pump blood OUT Diastole: occurs when the heart RELAXES after contraction Systole causes the ejection of blood into the aorta and pulmonary trunk. Systole, period of contraction of the ventricles of the heart that occurs between the first and the second heart sound Diastole period of relaxation of the heart muscle accompanied by the filing of the chambers with blood 15. Heart valves: When they open and close 4 valves of the heart 2 atrioventricular valves/ AV valves: tricuspid and bicuspid 2 semilunar valves/ SL : pulmonary and aortic Valves are responsible for unidirectional flow of blood through the heart The valves open and close in response to myocardial contractions and pressure changes within the heart As the RV contracts the TRICUSPID valve closes and the PULMONARY valve opens; closure of the TRUCUSPID valve keeps blood from backing up into the RIGHT ATRIUM and the opening of the PULMONARY valve allows blood to flow into the pulmonary artery into the Lung As the LV contracts the BICUSPID (mitral) valve closes, and the (SL) AORTIC valve opens, closure of the BICUSPID (mitral) valve prevents blood from backing into the LEFT ATRIUM and the opening of the AORTIC valve allows blood to flow into the aorta and into the Body 16. The production of heart sounds S1 and S2 : S1 is the first heart sound; caused by the closure of the bicuspid (mitral) valve and the tricuspid valves at the start of systole S2 is the second heart sound caused by the closure of the semilunar valves: the pulmonic and aortic valves marking the end of systole 17. Stenosis of the heart valves and effects; Aortic valve stenosis occurs when the heart and aortic valves narrows; the narrowing prevents blood flow from the heart into the aorta and into the body (during systole) systolic murmur crescendo and decrescendo heart murmur can be hears S/sx: abnormal heart sound and angina, chest pain Bicuspid (mitral)valve stenosis narrowing impairs blood flow from the L atrium to the L ventricle (most commonly due to rheumatic heart disease) low rumbling diastolic murmur at the apex radiating to the axilla hear during S1. s/sx of mitral valve stenosis: SOB, edema lower extremities, heart palpitations, chest pain dizziness, fatigue, coughing up blood 18. Stroke Volume: Stroke volume is determined by: Preload, Contractility, and Afterload Stroke volume is volume of blood which is about 70ml 19. Cor Pulmonale: Cor Pulmonale is abnormal enlargement of the right side of the heart as a result of the lungs or the pulmonary vessels: R side HF (Cor Pulmonale) is defined as the inability of the RV to provide adequate blood flow into pulmonary circulation: Causes: pulmonary disease, (Pulmonary HTN) the most common (or worsening L side HF) 20. Heart Failure: Heart failure is defined as a cardiac dysfunction caused by the inability of the heart to provide adequate cardiac output, resulting in inadequate tissue perfusion Left side heart failure is CHF the inability of the LV to provide adequate blood flow into the systemic circulation caused by HTN, Cardiac hypertrophy, or MI High systemic vascular pressure causes increase afterload and preload in the LV and LA, ultimately causing blood back up into the RV and RA causing back up of blood volume and pressure in the pulmonary veins= fluid into pulmonary capillaries into pulmonary tissues = pulmonary edema = dyspnea Right side heart failure if back into the vena cava and systemic veins= jugular distention, hepatosplenomegaly, and peripheral edema High Output heart failure HOF is caused by hyperthyroidism or nutritional deficiencies requiring an increased work load on the heart, at first the heart can keep up with the demands with adequate cardiac output and perfusion to tissues , but overtime will fail. 21. Hypertension: Patho: Consistent elevation of systemic arterial blood pressure Increased cardiac output and/or total peripheral resistance CO increased by any condition that increase HR and/or SV Peripheral resistance increases blood viscosity, reduce vessel diameter, vasoconstriction Primary HTN: essential or idiopathic (Genetic or environment) 92 to 95% of individuals with HTN have primary HTN Secondary HTN: HBP caused by effects of another dz (renal artery stenosis, kidney atrophy, increase in plasma renin) • There is a genetic vulnerability link to HTN in combination with environment, which contribute to the dysfunction of the sympathetic nervous system: renin-angiotensin- aldosterone RAA system and natueretic hormones as well as inflammation and insulin resistance • Insulin resistance and neurohumoral changes= sustained vasoconstriction and increased peripheral resistance • Inflammation contributes to renal dysfunction and increase blood volume • Increased peripheral resistance and increased blood volume are the 2 primary causes of sustained HTN Target organs for HTN are: eyes: retinal changes, kidney; renal dz, heart: CHF and CAD, brain: stroke or dementia 22. Calcium binding and troponin: Positively charged C ion is released from the sarcoplasmic reticulum: Ca has a affinity to bind with negatively charges troponin, this binding causes a physical shift of the tropomyosin (allowing exposure of the exposed myosin binding sites) the actin myosin proteins interact and the individual muscle fibers contract ATP IS REQUIRED TO FACILLITATE THIS INTERACTION Hematology: 23. Hematopoiesis: Hematopoiesis is blood cell formation Hematopoiesis site varies by age: it occurs throughout the lifespan Fetus: yolk sac to 3rd week gestation it is the initial site for hematopoiesis By 8 weeks gestation in the fetal liver and spleen By 5 months gestation in the bone marrow Birth to 5 years: red marrow of the bone After 20 years: red marrow of large bones: ilium, vertebrae, cranium, jaw, sternum, ribs, humerus, and femur Hematopoiesis is stimulated by: infiltration of yellow (fatty) bone marrow with red marrow cells- faster proliferation and differentiation of stem cells and daughter cells. 24. Risk factors for developing any type of anemia: Poverty Age Diet lacking in Fe vitamin B 12 and folic acid Menstruation Intestinal disorders: chrons or celiac Pregnancy Chronic conditions: Cancer, kidney dz, ulcers Family history: autoimmune disorders, alcoholism, exposure to toxic chemical, medication 25. Iron Deficiency Anemia: Iron deficiency anemia is a : microcytic hypochromic disorder= small cells low Hgb level Most common problem contributing to IDA is insufficient Fe availability Cause: inadequate dietary intake and chronic/occult bleeding (hemorrhage, GI ulcers, menorrhagia, colitis, cirrhosis, esophageal lesions) Patho: insufficient Fe levels or inability for mitochondria to utilize Fe effectively= decrease in Hgb synthesis= smaller/paler cells (2-4ml {1tsp} blood loss per day – 1-2mg Fe) 26. Erythrocyte function and lifespan: Primary function id gas exchange: to transport O2 bound to Hgb molecules, also to transport small amount of CO2 Lifespan 100-120 days 27. Sickle cell anemia: Sickle cell anemia; is a inherited disorder of the erythrocytes: Hemoglobinopathies: inherited autosomal recessive genetic disorder Patho: single amino acid change on the beta chain (valine replaces glutamic acid) leads to elongated Hgb molecules which does not bind to O2 readily= oxidative stress occurs: anxiety, fever, cold, dehydration: which further decreases O2 binding to Hgb and increases sickling tendency of the Hgb. The sickling of millions of Hgb molecules = distortion of RBC = weakening of RBC= rupture in 10 to 15 days This type of hemolytic anemia presents with classic s/sx of anemia with the addition of more serious complications: lysis of large amount of RBC puts sickle cell patients at risk for circulatory Fe overload * the abnormal RBCs also occlude: cerebral, splenic, and glomerular blood vessels.= increased risk of CVA (stroke), Splenic damage, or kidney damage Damage to the spleen is more prevalent; many sickle cell patients are asplenic before adulthood In persons of African descent 28. Thalassemia: Thalassemia is caused by an inherited autosomal recessive genetic disorder Patho: single or multiple amino acid changes on alpha and/or beta chains= synthesis of Hgb with abnormal chains or even missing alpha and beta chains *Depending on the mutation varying degrees of distortions or dysfunctions of RBC may occur* Due to the # of possible mutations Thalassemia can range from the very minor /asymptomatic to the most severe/being lethal such as (Cooley’s Thalassemia) In persons of Mediterranean or Southeastern Asia descent 29. Pernicious Anemia: Pernicious Anemia is a Macrocytic Normochromic anemia = Unusually large cells and normal Hgb level Problem: not enough building blocks available (folic acid and Vit B12) to make DNA so erythroblasts continue to grow larger instead of going through cell division Pernicious anemia is r/t Autoimmune Reaction: 90% of people have an Ab serum against GI tissue • As a genetic induced condition (English, Irish, or Scandinavian descent) • Autoimmune condition acquired secondary to GI infections; particularly H. Pylori • Pernicious anemia can also result from gastritis or gastrectomy Gastrectomy procedures result in the loss of GI cells : GI cells produce intrinsic factor (IF) the protein needed for Vit B12 absorption. The increase in bariatric surgeries has increased the prevalence of pernicious anemia Patho: malabsorption of B12 due to decrease IF production or secretion in the GI tract. Inadequate B12 levels result in decrease DNA synthesis and reduction of # RBC, but also decrease in nerve cell myelination = neuropathies associated with PA Unfortunately, w/o adequate IF to help with GI absorption, this type of anemia is hard to remedy by simple oral Vit B12, one cannot consume enough Vit B12 orally to force GI absorption: Vit B12 injection and nasal spray is the most effective way to get Vit B12 into the bloodstream 30. Hemolytic Anemia: Hemolytic anemia: literally mean “lysis” of blood cells Cause: Infection :(including parasitic or helminthic organisms, hemolytic toxin producing strains of Escherichia Coli. {found in food poisoning outbreaks} Transfusion reaction: from incorrect or incompatible blood Hemolytic dz of the newborn: Rh- Mom and Rh+ newborn Autoimmune reaction: congenital or idiopathic Drug Induced: Patho: premature destruction due to enzymes/toxins produced by infectious agents, mediated by own immune system, or the effects of certain chemicals or drugs 31. Erythropoetin: Erythropoetin is a growth factor made in the kidney and to a lesser degree the liver in response to tissue hypoxia Erythropoetin binds to the hemocytoblast and triggers a series of genetic and enzymatic changes with the proteogenic cell, setting it on course to develop into a mature erythrocyte 32. Function of hemoglobin: Hemoglobin has a unique chemical composition- polypeptide chains, heme molecules and iron – and can bind with 4 O2 molecules Hemoglobin plays an essential role in tissue oxygenation Hgb is contained in RBC, which efficiently carries O2 from the lung to the tissue of the body Hgb also helps to transport CO2 and H back to the lungs 33. Development of anemia due to gastrectomy: Gastrectomy procedures result in a loss of GI cells that produce IF the protein necessary for Vit B12 absorption Inadequate B12 levels= decrease DNA synthesis and reduction in RBC # Erythroblast continue to enlarge instead of divide= Pernicious Anemia 34. Effect of being transfused with the incorrect blood type: Hemolytic transfusion reaction occurs= development of hemolytic anemia “lysis” of the RBCs = the immune system attacks the transfused RBC / can be life threatening Genitourinary/ Renal: 35. Anatomy and physiology of the kidney: Kidney consists of 3 distinct area: Renal cortex: outer Renal medulla: middle Renal pelvis: inner The internal anatomy of the kidney: 2 distinct regions Outer renal cortex inner medulla- the medullary region consists of a # of cone shaped areas called renal pyramids The renal cortex & renal pyramids compose the parenchyma (the parenchyma is the functional potion of the kidney) within the parenchyma are microscopic structures called nephrons. Nephrons are the functional unit of the kidney= ultrafiltration unit for filtrate from the glomerulus (each kidney has 1 million nephrons) the Nephron is a multicellular structure consisting of: Bowman’s capsule Tubule system: proximal convoluted tubule (PCT), loop of Henle, DCT, and collecting ducts 2 capillary beds surround each Nephron the glomerulus capillary bed nestled in Bowman’s capsule and peritubular capillary surrounding the tubule system. Function of the kidney is to : maintain homeostasis, filter blood and produce urine, maintain ph, maintain BP, and eliminate waste (urea) as urine Four processes occur in the nephron during the formation of Urine; filtration, reabsorption, secretion and excretion As blood enter the glomerulus from the renal arterioles the process of glomerular fit ration occurs: BP forces water and dissolved plasma components (glucose, ions, amino acids, urea and creatinine) -what we now call “filtrate” through the glomerulus and Bowman’s capsule and into the renal tubule system (the filtration system dumps quite a bit of material into the tubule system*remember* anything that enters the tubule system could potentially be eliminated in the urine So, to prevent that reabsorption occurs. There is tubular reabsorption which is selective return of water and solutes (glucose, ions, amino acids, urea) FROM the filtrate of the nephron tubule system INTO the bloodstream of the peritubular capillaries. there are substances in our blood plasma that DO need to be eliminated from the body. But, because of either their size and/or chemical composition, these substances cannot be filtered through Bowman’s capsule. These substances remain in the glomerular capillaries and move into the efferent arteriole system, which ultimately connect to the peritubular capillaries. That leads to the next process: tubular secretion, the movement of material (urea, NH3, H+, K+, some Rx, misc. chem) FROM the bloodstream of the peritubular capillaries INTO the filtrate of the nephron tubule system And now the nephron is ready for the last step: urinary excretion, the final elimination of wastes (urea, creatinine, NH3, H+) and excess H2O and ions and the filtrate is now called urine A direct branch of the abdominal aorta the renal artery -divides into 2 smaller vessels within the kidney -individual afferent arterioles -supply the blood to the glomerulus; efferent arterioles connecting to the peritubular capillaries & eventually back to the renal vein and to the inferior vena cava 36. Nephron damage: Most kidney disease attacks the nephrons; causing them to lose their filtering ability. Damage to a nephron may happen quickly, often as the result of injury or poisoning; the 2 most common causes of kidney disease are HTN and diabetes You are born with all the nephrons you will ever have Nephron # decrease as a part of aging Nephron loss can be accelerated by factors Nephrons cannot regenerate: Nephron loss is permanent Nephron damage will cause a increase in creatinine levels Change renal vasculature Causes shunting b/t efferent and afferent arterioles 37. Tubular reabsorption and Tubular secretion: Tubular reabsorption: selective return of H2O and solutes (glucose, ions, amino acids, urea) FROM the filtrate of the nephron tubule system INTO the bloodstream of the peritubular capillaries. (these are capillaries that surround the tubular area of each nephron; reabsorption takes place throughout the tubules However, the PCT is the area that is most active 65% of salt and H2O and most organic substances including glucose and amino acids are reabsorbed in the PCT; the remainder of H2O and ions are reabsorbed throughput the tubule system with ADH and Aldosterone influencing the specific amounts) Tubular secretion: movement of material (urea, ammonia, hydrogen, potassium, some Rx, misc. chemicals) FROM the bloodstream of the peritubular capillaries INTO the filtrate of the nephron tubule system (this is a important way for the body to eliminate not only waste and extra ions but also medication, misc. chemicals such as food additives, preservative, flavoring, and coloring agents) 38. Conditions associated with renal failure: Unresolved acute kidney injury HTN Diabetes Mellites Systemic lupus 39. Calculi blockage of the ureter: Kidney stones: specific cause unknown Risk factors: Men 2:1 greater than women Those secreting elevated levels of: Ca, Mag, Ammonia, Phosphate, uric acid, and cystine Low ph increased rid of uric acid stone High ph increased risk of calcium phosphate stone Calcium stones account for 70 to 80% of all renal stones Precipitates and stones less than 5mm often pass on their own through the UT Larger stones may not pass and may create flank pain, nausea, vomiting Stones 1cm or greater have little probability of passing on their own and will require intervention 40. Benign Prostatic hypertrophy: BHP is enlargement of the prostate gland: this condition becomes problematic as the prostate tissue compresses the urethra where it passes through the prostate= frequency of lower urinary track symptoms. And can lead to partial or complete obstruction. BPH can cause AKI post renal 41. Prerenal, intrarenal and postrenal disease and what causes it: 3 types of Acute Kidney Injury Prerenal Intrarenal Postrenal Prerenal I which is caused by renal hypoperfusion due to severe prolonged hypotension, decreased CO, and or decreased blood volume or flow ( hemorrhage or severe dehydration) *most cases of AKI are prerenal* Intrarenal: which is caused by acute tubular necrosis (ATN) : acute glomerulonephritis ot other glomerulopathies: 50% of ATN occur postoperatively but can be r/t to burns, sever sepsis, or trauma; or as a result of contrast chemical esp., older adult) Post renal: which are caused by UT obstruction could be related to BPH, calculi, inflammation or tumor 42. Glomerulonephritis: Acute glomerulonephritis is an inflammation of the glomerular caused by glomerular injury, including immunologic response, ischemia, free radicals, drugs, toxins, vascular disorder and infection. 2nd glomerulonephritis is r/t systemic dz. * glomerular capillaries can trap blood borne Ab & AgAB complexes lead to glomerulonephritis* Patho: begins with a triggering event or infection: commonly develops as a sequala to beat hemolytic Streptococcal infection-our immune system responds to infection and involves formation of Ab and AgAb complexes form and deposit into glomerulus (not all phagocytized) leads to activation of complement system and WBC infiltration and coagulation cascade activation and fibrin deposition: which leads to glomerular injury and leakage and decreased capillary perfusion: resulting in proteinuria and hematuria and decreased GFR resulting in : proteinuria, hematuria, edema, increased creatinine, azotemia, and oliguria = Renal failure Clinical indicators of Glomerulonephritis: Proteinuria: loss of albumin from bloodstream and decreased oncotic pressure Hematuria Edema: decreased oncotic pressure Increased creatinine levels in serum Azotemia: presence of nitrogenous waste in plasma Oliguria: glomerular structure damaged to the point that nephrons no longer function as a ultra- fine filtration unit- urine output diminishes 43. Treatment for renal failure: there is no specific treatment for AKI: it requires individualized therapy and monitoring: primary goal of therapy once AKI has occurred is: maintain life until renal function restored: management principles directly r/t physiologic alterations: Correct fluid electrolyte imbalances Manage BP Prevent or treat infection Maintain nutrition Remember certain medications and their metabolites are not excreted and may be toxic In Advanced renal failure treatment is: dialysis or transplant 44. Blood hydrostatic pressure: the pressure exerted by the water in the blood against the inside wall of the bowman’s capsule: essential for BP in the glomerulus High hydrostatic pressure of the glomerular beds facilitates: filtration High oncotic pressure in the peritubular capillary beds and osmotic pressure tubular system facilitates: absorption 45. Kidney filtration: nephrons are the functional unit within the kidney =ultrafine filtration unit for filtrate from the glomerulus. As blood enters the glomerulus from the renal arteriole system, the process of glomerular filtration occurs BP forces– water and dissolved plasma (glucose, ions, amino acids, urea, creatinine) the filtrate through the glomerulus and bowman’s capsule and into the renal tubule system: through tubular reabsorption then moves on to tubular secretion then excretion 46. Role of angiotensin converting enzyme ACE: when renin is released it cleaves to a globulin into the plasma to form Angiotensin 1 which is physiologically inactive, in the presence of ACE (produced from the pulmonary and renal endothelium) Angiotensin 1 is converted to Angiotensin 2 Angiotensin 2 stimulates secretion of aldosterone by adrenal cortex is a potent vasoconstrictor and stimulates ADH secretion and thirst… Lowers urine protein excretion and controls BP 47. Role of the Macrophage: macrophage plays a significant part in immunity and immune response: Defensive role- carrying out phagocytosis Regulates lymphocyte activation and proliferation Essential in activation process of T and B lymphocytes A macrophage is a type of phagocyte which is a cell responsible for detecting, engulfing, and destroying pathogens and apoptic cells Macrophages are produced through differentiation of monocytes which turn into macrophages when they leave the blood [Show More]
Last updated: 1 year ago
Preview 1 out of 12 pages
Instant download

Buy this document to get the full access instantly
Instant Download Access after purchase
Add to cartInstant download
Reviews( 0 )
Document information
Connected school, study & course
About the document
Uploaded On
Oct 21, 2022
Number of pages
12
Written in
Additional information
This document has been written for:
Uploaded
Oct 21, 2022
Downloads
0
Views
33