Material Science > QUESTIONS & ANSWERS > Test 3 prep - Stony Brook University ESG 332 (All)
Test 3 prep - Stony Brook University ESG 332
Document Content and Description Below
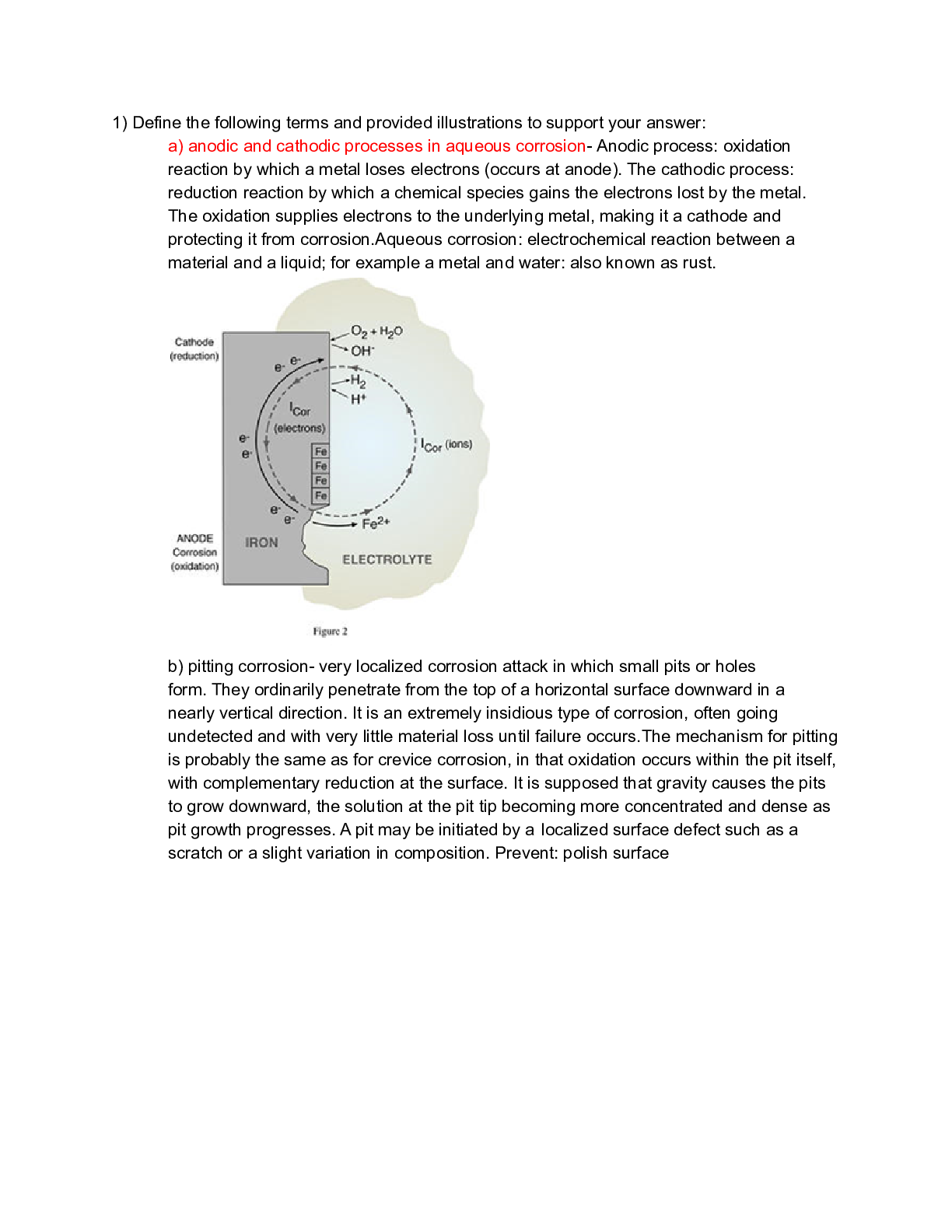
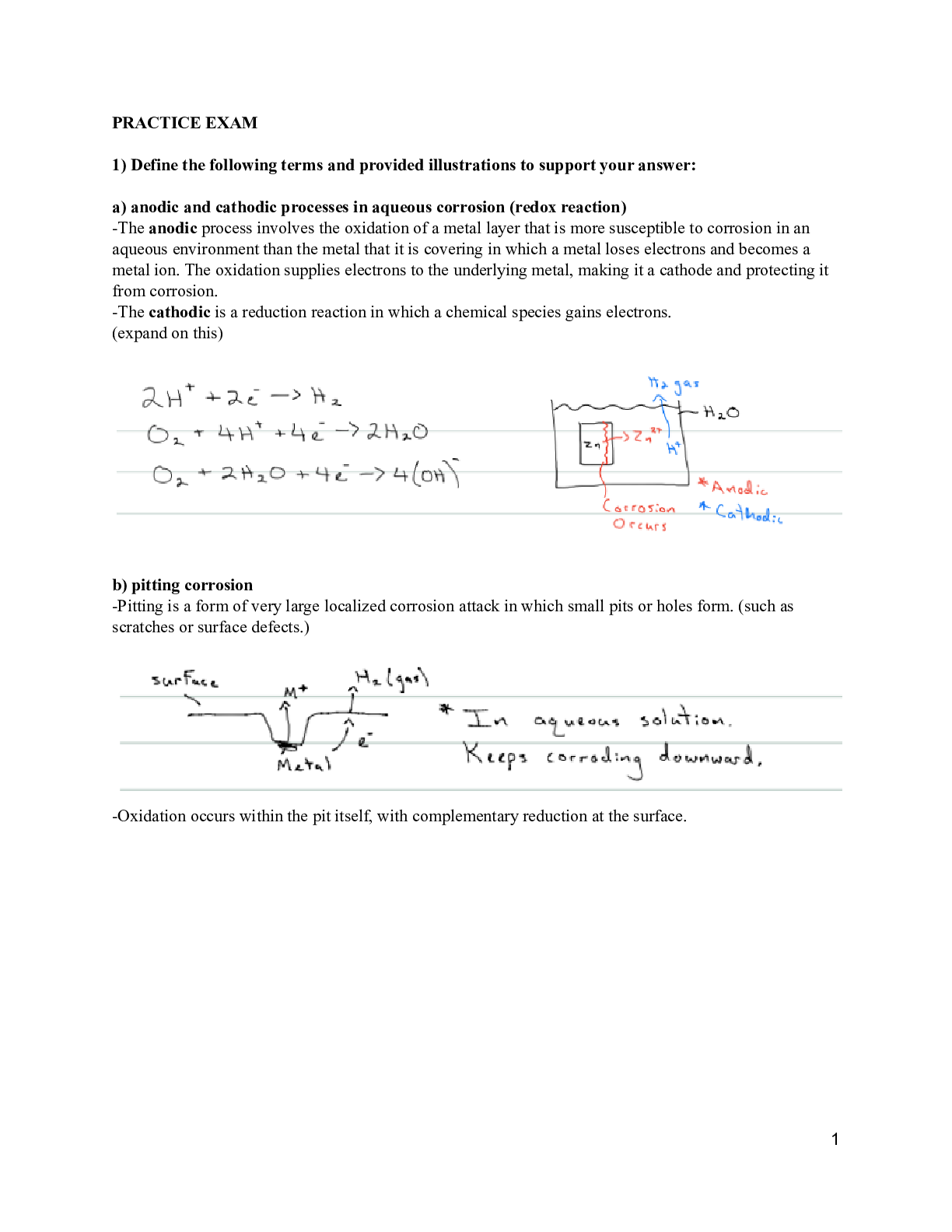
1) Define the following terms and provided illustrations to support your answer: a) anodic and cathodic processes in aqueous corrosion- Anodic process: oxidation reaction by which a metal loses elec... trons (occurs at anode). The cathodic process: reduction reaction by which a chemical species gains the electrons lost by the metal. The oxidation supplies electrons to the underlying metal, making it a cathode and protecting it from corrosion.Aqueous corrosion: electrochemical reaction between a material and a liquid; for example a metal and water: also known as rust. b) pitting corrosion- very localized corrosion attack in which small pits or holes form. They ordinarily penetrate from the top of a horizontal surface downward in a nearly vertical direction. It is an extremely insidious type of corrosion, often going undetected and with very little material loss until failure occurs.The mechanism for pitting is probably the same as for crevice corrosion, in that oxidation occurs within the pit itself, with complementary reduction at the surface. It is supposed that gravity causes the pits to grow downward, the solution at the pit tip becoming more concentrated and dense as pit growth progresses. A pit may be initiated by a localized surface defect such as a scratch or a slight variation in composition. Prevent: polish surface c) intergranular corrosion- occurs preferentially along grain boundaries for some alloys and in specific environments. The net result is that a macroscopic specimen disintegrates along its grain boundaries. This type of corrosion is especially prevalent in some stainless steels. When heated to temperatures between 500C and 800C (950F and 1450F) for sufficiently long time periods, these alloys become sensitized to intergranular attack. This heat treatment permits the formation of small precipitate particles of chromium carbide (Cr23C6) by reaction between the chromium and carbon in the stainless steel. Both the chromium and the carbon must diffuse to the grain boundaries to form the precipitates, which leaves chromium-depleted zone adjacent to the grain boundary. Consequently, this grain boundary region now highly susceptible to corrosion. Prevent: heat treatment (redissolve), lower carbon, diff alloy. d) atmospheric oxidation of metal- an oxide layer or scale forms on the surface of the metal. Oxide layer increases in thickness electrons be conducted to the scale–gas interface, at which point the reduction reaction occurs; in addition, M2+ ions must diffuse away from the metal–scale interface, and/or O2- ions must diffuse toward this same interface e) crevice corrosion- Electrochemical corrosion may also occur as a consequence of concentration differences of ions or dissolved gases in the electrolyte solution and between two regions of the same metal piece. For such a concentration cell, corrosion occurs in the locale that has the lower concentration. A good example of this type of corrosion occurs in crevices and recesses or under deposits of dirt or corrosion products where the solution becomes stagnant and there is localized depletion of dissolved oxygen. The crevice must be wide enough for the solution to penetrat [Show More]
Last updated: 1 year ago
Preview 1 out of 10 pages
Instant download
Instant download
Reviews( 0 )
Document information
Connected school, study & course
About the document
Uploaded On
Dec 13, 2022
Number of pages
10
Written in
Additional information
This document has been written for:
Uploaded
Dec 13, 2022
Downloads
0
Views
40


.png)



.png)


.png)






.png)