*NURSING > EXAM > NURN 110 Nursing Process Focus: Patients Receiving Hydrochlorothiazide,100% CORRECT (All)
NURN 110 Nursing Process Focus: Patients Receiving Hydrochlorothiazide,100% CORRECT
Document Content and Description Below
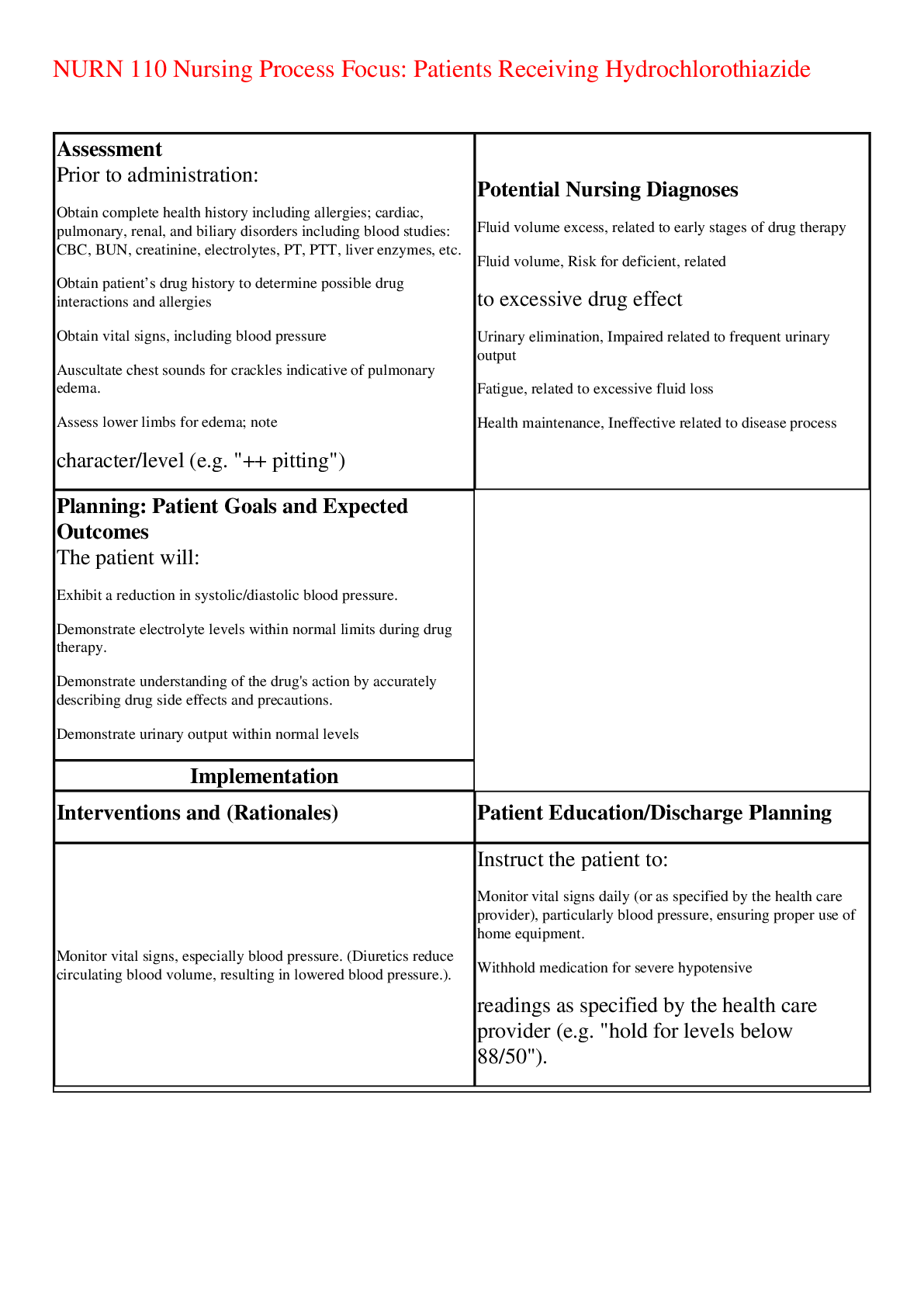
NURN 110 Nursing Process Focus: Patients Receiving Hydrochlorothiazide Assessment Prior to administration: • Obtain complete health history including allergies; cardiac, pulmonary, renal, and b... iliary disorders including blood studies: CBC, BUN, creatinine, electrolytes, PT, PTT, liver enzymes, etc. • Obtain patient’s drug history to determine possible drug interactions and allergies • Obtain vital signs, including blood pressure • Auscultate chest sounds for crackles indicative of pulmonary edema. • Assess lower limbs for edema; note character/level (e.g. "++ pitting") Potential Nursing Diagnoses • Fluid volume excess, related to early stages of drug therapy • Fluid volume, Risk for deficient, related to excessive drug effect • Urinary elimination, Impaired related to frequent urinary output • Fatigue, related to excessive fluid loss • Health maintenance, Ineffective related to disease process Planning: Patient Goals and Expected Outcomes The patient will: • Exhibit a reduction in systolic/diastolic blood pressure. • Demonstrate electrolyte levels within normal limits during drug therapy. • Demonstrate understanding of the drug's action by accurately describing drug side effects and precautions. • Demonstrate urinary output within normal levels Implementation Interventions and (Rationales) Patient Education/Discharge Planning • Monitor vital signs, especially blood pressure. (Diuretics reduce circulating blood volume, resulting in lowered blood pressure.). Instruct the patient to: • Monitor vital signs daily (or as specified by the health care provider), particularly blood pressure, ensuring proper use of home equipment. • Withhold medication for severe hypotensive readings as specified by the health care provider (e.g. "hold for levels below 88/50"). • Observe for changes in level of consciousness, dizziness, fatigue, postural hypotension. (Reduction in circulating blood volume may produce changes in level of consciousness or syncope.) Instruct the patient to • Immediately report any change in consciousness, especially feeling faint. • Avoid abrupt changes in posture; rise slowly • from prolonged periods of sitting/lying down. • Obtain blood pressure readings in sitting, standing and supine positions to monitor fluctuations in blood pressure. • Monitor for fluid overload and signs of congestive heart failure, including Instruct the patient to: • Immediately report any severe shortness of electrolyte balance, intake and output, and daily weights. • (Increased blood volume causes increased cardiac workload and pulmonary edema.) breath, frothy sputum, profound fatigue and edema in extremities, potential signs of heart failure or pulmonary edema • Accurately measure intake, output, body weight; measure and weigh daily. • Evaluate "insensible loss" occurring from sweat, etc; avoid excessive heat, which contributes to increases in insensible loss. • Consume adequate amounts of plain water • Remain adequately but not over-hydrated. that increased urine output and decreased weight indicate that the drug is working. • Monitor nutritional status. (Electrolyte imbalances may be counteracted by dietary measures.) Instruct patients to: • Take medication with a glass of orange juice to promote adequate potassium intake. • Use sparing amounts of "salt substitute" (potassium chloride) to season foods. • Avoid excessive use (20 grams or one half oz.) of natural black licorice (licorice root), which can reduce potassium levels. • Consult with health care provider before using unprescribed vitamin/mineral supplements or electrolyte-fortified sports drinks. • Use with caution in diabetics. Observe for signs of hyperglycemia. (Hydrochlorothiazide alters glycemic control and may cause hyperglycemia.) • Provide patients with written materials on diabetes mellitus signs, symptoms and treatment of diabetes mellitus • Monitor renal and metabolic function via laboratory tests such as CBC, BUN, creatinine, uric acid level, serum electrolytes and glucose, cholesterol, triglycerides, and complete liver panel including enzymes. (Hydrocholorothiazide is metabolized in the liver and excreted by the kidneys; impaired organ function can increase serum drug levels. • Hydrochlorothiazide may increase the following levels: serum glucose, triglycerides, cholesterol, uric acid, BUN, creatinine and calcium. This drug may Instruct the patient or caregiver to: • Immediately report signs and symptoms of metabolic imbalances: o nausea and vomiting o profound weakness o Lethargy o muscle spasms or cramps o depression/ disorientation o hallucinations o palpitations/ irregular heart beat o numbness or tingling in limbs o paralysis o extreme thirst o extreme increase or decrease in urine output • Adhere to regimen of laboratory testing as ordered by the health care provider. decrease the following levels: sodium, potassium, chloride may cause metabolic alkalosis.) • Observe for hypersensitivity reaction\, or signs of infection. Instruct the patient or caregiver to: • Report any difficulty breathing, throat tightness, hives or rash or bleeding. • Report any "flu-like" symptoms: shortness of breath, fever, sore throat, malaise, joint pain, profound fatigue, etc. • Observe for signs of pancreatitis or hepatic toxicity. (These are potential side effects of drug.) Instruct the patient to: • Report any frank bleeding, severe epigastric or abdominal pain, anorexia, heartburn, nausea, vomiting, jaundice or a change in the color or character of stools. • Take the drug with milk or food if stomach upset occurs. • Ensure patient safety. (Dizziness or syncope caused by postural hypotension increases the risk of fall injuries.) Instruct the patient to: • Call for assistance before getting out of bed or attempting to ambulate alone. • Avoid sudden changes of position to prevent • dizziness caused by postural hypotension. • Avoid driving or other activities requiring mental alertness or physical agility until blood pressure is stabilized and effects of the medication are known. • Monitor reactivity to light exposure (photosensitivity). • Instruct patient to limit exposure to the sun, • wear dark glasses, and light colored loose- fitting clothes when outdoors. • Use cautiously with the elderly. (Symptoms that might be more pronounced in the elderly are hypotension and dysrhythmias. Such effects may be caused by increased serum drug levels related to diminished kidney and liver function as a result of aging. Elderly patients may require lower dosages.) • Instruct the patient or caregiver to keep a symptom log during the initial phase of therapy to assist the health care provider in tailoring dosages as needed. Evaluation of Outcome Criteria Evaluate the effectiveness of drug therapy by confirming that patient goals and expected outcomes have been met (see “Planning”). Nursing Process Focus: Patients Receiving Nifedipine Assessment Prior to administration: • Obtain complete health history including possible drug interactions and allergies • Obtain EKG and vital signs, including blood pressure. • Assess neurological status and level of consciousness. • Auscultate chest sounds for rales or rhonchi ("crackles") indicative of pulmonary edema. • Assess lower limbs for edema; note Potential Nursing Diagnoses • Health Maintenance, ineffective related to disease process • Knowledge Deficient, related to drug action and side effects • Cardiac output, decreased, related to inadequate contractility • Tissue perfusion, Ineffective related to decreased cardiac output Planning:Patient Goals and Expected Outcomes The patient will: • Exhibit a reduction in systolic/diastolic blood pressure. • Patient will demonstrate understanding of the drug's action by accurately describing drug side effects and precautions. • Demonstrate adequate tissue perfusion Implementation Interventions and (Rationales) Patient Education/Discharge Planning • Monitor vital signs, especially blood pressure and heart rate. Have EKG conducted during intial therapy. (Nifedipine dilates the main coronary arteries and arterioles, reducing blood pressure.) Instruct the patient to • Monitor vital signs regularly, particularly blood pressure, ensuring proper use of home equipment. • Withhold medication for severe hypotensive readings as specified by the health care provider (e.g. "hold for levels below 80/50"). • Immediately report palpitations or rapid heart beat. • Observe for changes in level of consciousness, dizziness, fatigue, postural hypotension, increased chest pain (caused by vasodilation and/or hypotension). Instruct the patient to immediately report to: • Any change in consciousness particularly sense of faintness. • Increase or return of chest pain or other angina-like symptoms Instruct patient to: • Avoid abrupt changes in posture; rise slowly from prolonged periods of sitting/lying down. • Obtain blood pressure readings in sitting, standing and supine positions to monitor fluctuations in blood pressure. • Monitor fluid and electrolyte balance. • Monitor for signs of congestive heart failure (CHF), an adverse reaction. (Edema is a side-effect of nifedipine.) • Instruct the patient to immediately report any severe shortness of breath, frothy sputum, profound fatigue and swelling which may be signs of heart failure or pulmonary edema. • Monitor weight, intake and output, and for fluid accumulation. (Nifedipine decreases myocardial contractility, increasing the risk of CHF.) Instruct patient to: • Accurately measure intake, output daily, body weight; • Avoid excessive heat which contributes to increases in insensible loss. • Consume adequate amounts water • Observe for hypersensitivity reaction. • Instruct the patient or caregiver to immediately report: difficulty breathing, throat tightness, hives or rash muscle cramps or tremors . • Monitor liver and kidney function via laboratory tests. (Nifedipine is metabolized in the liver and excreted by the kidneys.) Instruct the patient to report: • Signs of hepatotoxicity: nausea, vomiting, anorexia, bleeding, • Severe epigastric or abdominal pain, heartburn, jaundice or a change in the color or character of stools. • Signs of renal toxicity: fever, flank pain, changes in urine output, color or character (eg. cloudy, with sediment, etc.) Instruct patient to • adhere to laboratory testing regimen for serum blood level tests of liver enzymes as directed. by the health care provider • Observe for constipation. (Decreased fluid intake may lead to constipation.) Advise the patient to: • Maintain adequate exercise and fluid intake. • Consider using a bulk laxative or stool softener as recommended by the health care provider. • Ensure patient safety. (Monitor ambulation due to the risk of injury from possible changes in sensorium related to hypotension.) • Instruct patient to avoid activities that require mental alertness and physical coordination until effect of drug is known. • Use cautiously with the elderly. (Symptoms that might be more pronounced in the elderly are hyportension and dysrhythmias. Elderly patients may require lower dosages.) • Instruct the patient or caregiver to keep a blood pressure and symptom (headache, dizziness, etc.) log during the initial phase of therapy to assist the health care provider in tailoring dosages as needed. Evaluation of Outcome Criteria Evaluate the effectiveness of drug therapy by confirming that patient goals and expected outcomes have been met (see “Planning”). Nursing Process Focus: Patients Receiving Enalapril Assessment Prior to administration: • Obtain complete health history including allergies, drug history to determine possible drug interactions and allergies; identify any history of angioedema. • Collect specimens for: CBC, BUN, creatinine, electrolytes, liver enzymes, etc. • Obtain EKG and vital signs, including blood pressure. • Assess neurological status and level of consciousness. Potential Nursing Diagnoses • Injury, Risk for related to falls • Knowledge Deficient, related to drug action and side effects • Tissue perfusion, Ineffective related to decreased cardiac contractility. • Nutrition: more than body requirement, risk for imbalanced related to hyperkalemia. Planning: Patient Goals and Expected Outcomes The patient will: • Exhibit a reduction in systolic/diastolic blood pressure. • Demonstrate serum electrolyte levels within normal limits during drug therapy. • Demonstrate understanding of the drug's action by accurately describing drug side effects and precautions. • Remain free from injury related to falls Implementation Interventions and (Rationales) Patient Education/Discharge Planning • Monitor vital signs, especially signs of hypotension. (Enalapril can produce "first dose phenomenon" of profound hypotension.) Instruct the patient • That changes in consciousness may occur due to rapid reduction in blood pressure • To immediately report dyspnea, difficulty swallowing, itching, or impending syncope. • of African descent of the changes in efficacy and increased risk of angioedmea (angioedema) associated with ACE inhibitors such as enalapril. • About the first dose phenomenon; reassure that this effect diminishes with continued therapy. • That enalapril takes effect in approximately an hour and peaks in three to four hours; rest in the supine position beginning one hour after administration and for three to four hours after the first dose. • To always arise slowly, avoiding sudden posture changes. • Observe for hypersensitivity reaction, particularly angioedema. Instruct the patient or caregiver to: • Immediately report any difficulty breathing, • hoarseness, throat tightness, "thick tongue," hives or rash. These symptoms can occur upon the first dose or much later as a delayed reaction. • Call emergency services for severe dyspnea or hoarseness accompanied by swelling of the face or mouth because Angioedema can be life-threatening. • Observe for signs of infection which may indicate insidious onset of blood dyscrasia: fever, sore throat, malaise, joint pain, ecchymoses, profound fatigue, shortness of breath, pallor, etc. (Bruising is a sign of bleeding which can also indicate the presence of a serious blood disorder.) • Instruct patient to immediately report any "flu-like" symptoms: flu-like symptoms may herald the "silent" onset of serious blood disorder, such as neutropenia or agranulocytosis. • Monitor neurological status for dizziness, drowsiness or lightheadedness. (These are signs of decreased blood flow to the brain due to the drug’s vasodilating hypotensive action.) Instruct patient to: • Report dizziness or syncope which persists beyond the first dose, as well as paraesthesias and other neurological changes. • Contact the health care provider immediately if syncope occurs.(before the next scheduled dose of the drug) if syncope occurs. • Monitor for persistent dry cough, triggered by bradykinin's pro-inflammatory action changes in cough pattern, and serious paroxysms of cough. (Cough results from reaction by the mucous membranes in the throat and trachea. May indicate respiratory swelling and angioedema). • . Inform the patient: • That persistent dry cough may be expected. • To distinguish the difference between expected cough and cough of a more serious nature (such as accompanied by fever or shortness of breath) • Any changes in the character or frequency of cough, such becoming productive or occurring upon exertion must be reported. • Cough accompanied by chest, arm, or back pain or pressure could signal a heart attack. Any cough accompanied by shortness of breath, fever or chest discomfort should be reported immediately. • Cough may be more troublesome when in supine position; sleeping with the head elevated may be more comfortable. • Cough can sometimes be relieved by using non-medicated lozenges or hard candies. • (Diabetics may use sugar-free hard candies.) • Monitor effectiveness of drug therapy. • Document changes in blood pressure and pulse in response to drug administration. Instruct the patient to: • Take BP and pulse in both arms, daily, while lying, sitting and standing or as often as specified by the health care provider. • regarding the normotensive range of blood pressure; instruct the patient to consult the health care provider regarding "reportable" blood pressure readings (e.g. "lower than 80/50). • Keep a symptom (e.g. dizziness, etc.) and blood pressure diary during initial and/or dosage adjustment phases of therapy. • Monitor fluid and electrolyte balance (see following box). • Monitor for dehydration or fluid overload. (Dehydration causes low circulating blood volume and will exacerbate hypotension. • Severe dehydration may trigger syncope and collapse. Pitting edema is a sign of fluid retention, and can be a sign of CHF, and may indicate reduced drug efficacy.) Instruct the patient to: • Observe for signs of dehydration such as oliguria, dry lips and mucous membranes, poor skin turgor, etc. • Report any bodily swelling which may indicate angioedema or fluid retention; note any pitting edema. • Accurately measure intake, output, body weight; measure and weigh daily. • Monitor increased need for fluid caused by vomiting, diarrhea or excessive sweating. avoid excessive heat which can increase insensible fluid loss. • Consume adequate amounts of plain water • Remain adequately but not over-hydrated. • Monitor for hyperkalemia. (Hyperkalemia occurs due to reduced aldosterone levels.) • . Instruct the patient to: • Immediately report signs of hyperkalemia: nausea, irregular heartbeat, profound fatigue/ muscle weakness, and slow or faint pulse. • Avoid consuming electrolyte-fortified "nutritional" snacks, or sports drinks which may contain potassium. • Avoid using salt substitute (KCL) to flavor foods. • Consult the health care provider before taking any nutritional supplements containing potassium. • Monitor for signs of hepatic or renal toxicity. • (Enalapril is metabolized by the liver and excreted by the kidneys. Impaired function Instruct the patient to • Immediately report the following: nausea, vomiting, diarrhea, rash, jaundice, abdominal results in increased serum drug levels.) • Observe for jaundice pain, tenderness or distention, or change in character and color of stool or urine, flank pain, hematuria. • Stop the drug immediately and contact the health care provider if jaundice occurs. • Adhere to a regular schedule of laboratory testing for liver and kidney function as ordered by the health care provider • Ensure patient safety. Monitor ambulation until response of the drug is known. Instruct the patient to • Obtain help prior to getting out of bed or attempting to walk alone. • Avoid activities that require mental concentration and physical agility until effect of drug is known. Evaluation of Outcome Criteria Evaluate the effectiveness of drug therapy by confirming that patient goals and expected outcomes have been met (see “Planning”). Nursing Process Focus: Patients Receiving Doxazosin Assessment Prior to administration: • Obtain complete health history, including allergies, history: cardiac, pulmonary, renal/urogenital, biliary, and mental or sleep disorders, including EKG and laboratory studies: CBC, BUN, creatinine, electrolytes, liver enzymes. • Obtain patient’s drug history to determine possible drug interactions and allergies. • Assess vital signs including heart sounds and EKG. Potential Nursing Diagnoses • Health Maintenance, Impaired related to disease process • Tissue Perfusion, Ineffective related to decreased contractility • Knowledge Deficient, related to drug action and side effects • Injury, Risk for, related to falls • Sexual dysfunction, Risk for related to side effect of medication • Noncompliance, Risk for related to drug side effects Planning: Patient Goals and Expected Outcomes The patient will • Exhibit a reduction in systolic/diastolic blood pressure. • Demonstrate understanding of the drug's action by accurately describing drug side effects and precautions. • Continue drug regimen as prescribed • Maintain usual sexual function Implementation Interventions and (Rationales) Patient Education/Discharge Planning • Ensure patient safety by monitoring ambulation until response of the drug is known. (Drug may cause drowsiness, hypotension.) Instruct the patient to • Call for assistance when getting out of bed or attempting to walk. • Remove any tripping hazards from the home environment. Drowsiness or dizziness increases the risk of falls. • Avoid activities that require mental alertness and physical coordination until effect of drug is known • Monitor vital signs and cardiovascular status. (In hypotensive emergencies, monitor all vital signs Instruct the patient to • Monitor vitals signs (especially blood pressure), ensuring proper use of home equipment. • Immediately report any palpitations, chest pain, weakness, numbness or tingling or other disturbing symptoms, etc. • Monitor neurological status, including level of consciousness and mood. (Observe carefully for dizziness, drowsiness or • Instruct patient to immediately report any dizziness, faintness or feelings of dysphoria. lightheadedness, which are signs of decreased blood flow to the brain due to the drug’s hypotensive action.) • Monitor emotional status. (Doxazosin can exacerbate existing mental depression due to its depressant action on the central nervous system). • Interview patient regarding suicide potential, obtain a "no-self harm" verbal contract from the patient • • Monitor genito-urinary and renal status. (Doxazosin relaxes smooth muscle in the prostate and bladder neck, reducing urethral resistance. The adrenergic blocking action of Doxazosin produces vasodilation in the penis, which may result in priaprism.) • Instruct the patient to immediately report any persistent painful erection (priaprism) to the health care provider. • Monitor fluid intake and (especially) urine output. • Instruct patient to report changes in urinary output to the health care provider • Monitor laboratory studies for kidney function: CBC, BUN, Uric Acid, Creatinine, urinalysis, etc. (Doxazosin is excreted by the kidneys.) • Ensure that patient understands the importance of keeping appointments for follow up lab studies • Monitor BP and pulse in both arms while patient is lying, sitting, and standing. Monitor BP 2-3 hours after dosing and at end of dosing interval to ensure maintained BP control. • Document changes in blood pressure and pulse in response to drug administration. • Instruct patient to monitor BP and pulse, in both arms while lying, sitting and standing, at regular intervals as advised. • Monitor liver function lab tests: PT, PTT, alkaline phosphotase, amylase, SGOT, SGPT, etc. (Doxazosin is metabolized in the liver.) • Instruct the patient to report signs and symptoms of hepatic toxicity: nausea, vomiting, diarrhea, rash, jaundice, abdominal pain, tenderness or distention, or change in color of stool, • Use cautiously with the elderly. • Inform the patient or caregiver that elderly adults may require lower dosages. Evaluation of Outcome Criteria Evaluate the effectiveness of drug therapy by confirming that patient goals and expected outcomes have been met (see “Planning”). Nursing Process Focus: Patients Receiving Hydralazine (Apresoline) Assessment Prior to administration: • Obtain complete health history inclujding allergies; cardiac, pulmonary, renal, and biliary disorders including blood studies: CBC, BUN, creatinine, electrolytes, PT, PTT, liver enzymes, etc. • Obtain patient’s drug history to determine possible drug interactions and allergies • Obtain EKG and vital signs, including blood pressure. • Auscultate heart and chest sounds • Assess neurological status and level of consciousness. Potential Nursing Diagnoses • Tissue perfusion, Ineffective related to constricted peripheral vessels • Knowledge deficient, related to drug action and side effects • Fluid volume, excess related to disease process Planning: Patient Goals and Expected Outcomes The patient will • Exhibit a reduction in systolic/diastolic blood pressure. • Demonstrate understanding of the drug's action by accurately describing drug side effects and precautions. • Demonstrate reduction in fluid volume Implementation Interventions and (Rationales) Patient Education/Discharge Planning • Use with caution with impaired cardiac/cerebral circulation. (The drop in blood pressure produced by hydralazine may further compromise individuals who already suffer from ischemia.) • Instruct the patient to immediately report: angina-like symptoms: chest, arm, back and/or neck pain, palpitations, faintness, dizziness, drowsiness, any sensation of cold, numbness, tingling, pale or dusky look to the hands and feet. headache or signs of stroke: facial drooping, visual changes, limb weakness or paralysis. • Monitor vital signs (especially blood pressure) daily or as often as advised by the health care provider. • Monitor for dizziness. (This is a sign of hypotension that occurs because the brain is not getting enough blood flow.) • Advise patient to use caution when driving, operating machinery, or performing other hazardous activities, especially until effects of the medication are known. Evaluation of Outcome Criteria Evaluate the effectiveness of drug therapy by confirming that patient goals and expected outcomes [Show More]
Last updated: 1 year ago
Preview 1 out of 15 pages
Instant download
Buy this document to get the full access instantly
Instant Download Access after purchase
Add to cartInstant download
Reviews( 0 )
Document information
Connected school, study & course
About the document
Uploaded On
Mar 24, 2023
Number of pages
15
Written in
Additional information
This document has been written for:
Uploaded
Mar 24, 2023
Downloads
0
Views
19














