Chemistry > QUESTION PAPER (QP) > AQA 2022// GCSE COMBINED SCIENCE: TRILOGY Foundation Tier Chemistry Paper 1F (All)
AQA 2022// GCSE COMBINED SCIENCE: TRILOGY Foundation Tier Chemistry Paper 1F
Document Content and Description Below
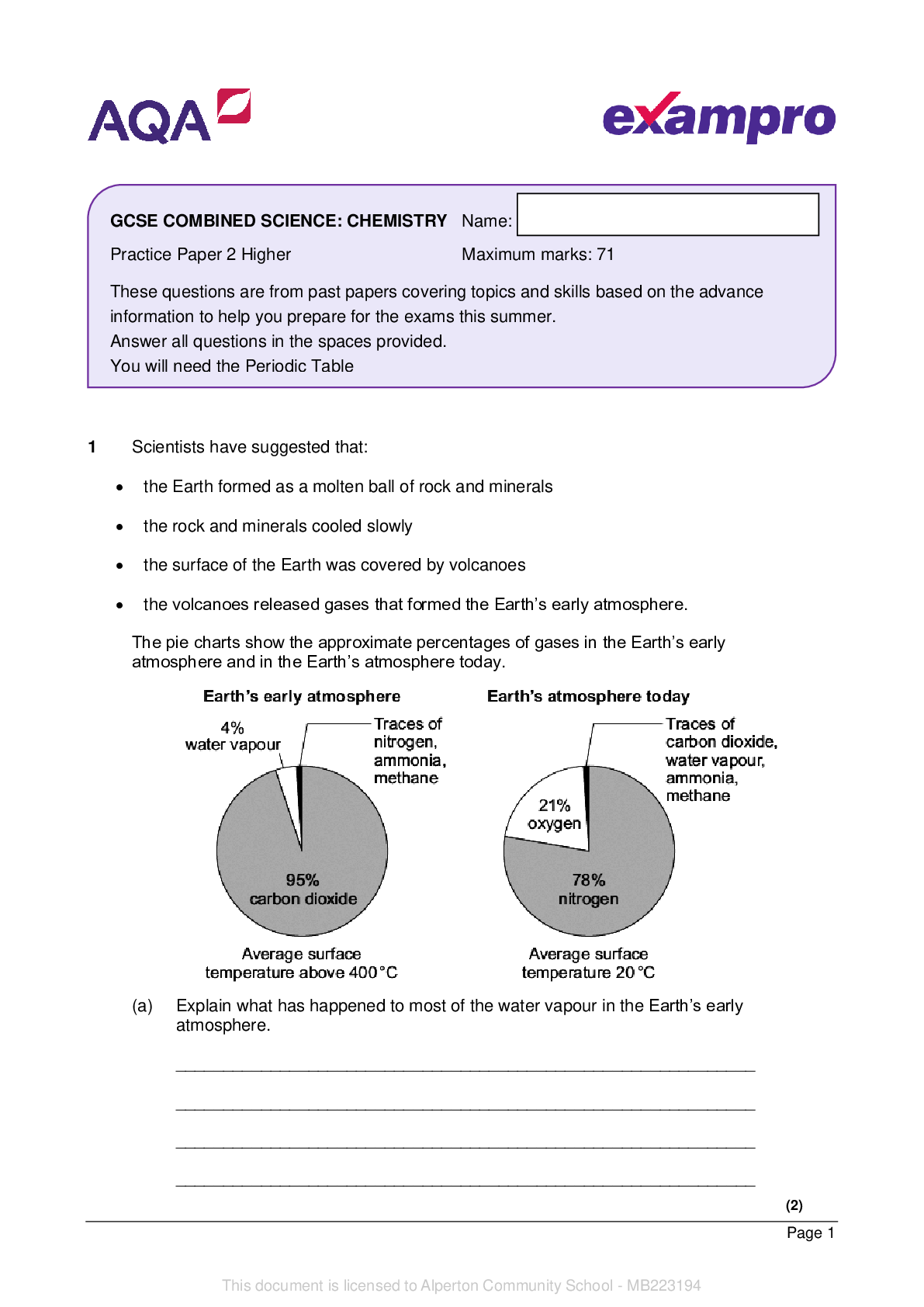
Materials For this paper you must have: • a ruler • a scientific calculator • the periodic table (enclosed). Instructions • Use black ink or black ball-point pen. • Pencil should only... be used for drawing. • Fill in the boxes at the top of this page. • Answer all questions in the spaces provided. • If you need extra space for your answer(s), use the lined pages at the end of this book. Write the question number against your answer(s). • Do all rough work in this book. Cross through any work you do not want to be marked. • In all calculations, show clearly how you work out your answer. Information • The maximum mark for this paper is 70. • The marks for questions are shown in brackets. • You are expected to use a calculator where appropriate. • You are reminded of the need for good English and clear presentation in your answers. Please write clearly in block capitals. Centre number Candidate number Surname Forename(s) Candidate signature I declare this is my own work. GCSE COMBINED SCIENCE: TRILOGY Foundation Tier Chemistry Paper 1F F 2 *02* IB/M/Jun22/8464/C/1F 0 1 This question is about Group 1 elements. 0 1 . 1 What are the Group 1 elements known as? [1 mark] Tick () one box. Alkali metals Halogens Noble gases 0 1 . 2 Figure 1 shows a lithium atom. Figure 1 What is the number of electrons and neutrons in the atom of lithium? [2 marks] Number of electrons Number of neutrons 0 1 . 3 What is the relative charge on a lithium ion? [1 mark] Tick () one box. +1 0 –1 Do not write outside the box 3 *03* Turn over ► IB/M/Jun22/8464/C/1F Do not write outside the 0 1 box . 4 Lithium is heated and then cooled in an experiment. Two physical changes happen in the experiment. Draw one line from each stage to the physical change that happens in that stage. [2 marks] Stage Physical change Stage 1 Stage 2 Boiling Condensing Dissolving Freezing Melting Question 1 continues on the next page 4 *04* IB/M/Jun22/8464/C/1F Do not write outside the Figure 2 box represents the melting points of some Group 1 elements. Figure 2 5 *05* Turn over ► IB/M/Jun22/8464/C/1F Do not write outside the 0 1 box . 5 What is the melting point of caesium? Use Figure 2. [1 mark] Melting point = °C 0 1 . 6 The melting point of potassium is 63 °C Draw a bar for the melting point of potassium on Figure 2. [1 mark] 0 1 . 7 Describe the trend of the melting points of the Group 1 elements in Figure 2. [3 marks] 0 1 . 8 The boiling point of sodium is 883 °C What is the state of sodium at 150 °C? Use Figure 2. [1 mark] Tick () one box. Gas Liquid Solid 6 *06* IB/M/Jun22/8464/C/1F Do not write outside the box 13 0 1 . 9 Figure 3 represents the electronic structure of a sodium atom and of a potassium atom. Figure 3 Complete the sentence. Choose the answer from the box. [1 mark] gains an electron loses an electron shares an electron Potassium is more reactive than sodium because potassium more easily . 7 *07* Turn over ► IB/M/Jun22/8464/C/1F Do not write outside the box 0 2 This question is about hydrogen chloride and sodium hydroxide. 0 2 . 1 A chlorine atom has 7 electrons in the outer shell. A hydrogen atom has 1 electron in the outer shell. Figure 4 represents part of a dot and cross diagram for a molecule of hydrogen chloride. Complete the dot and cross diagram. Use dots (ο) and crosses (x) to represent electrons. You should show only the electrons in the outer shells. [2 marks] Figure 4 0 2 . 2 Hydrogen chloride dissolves in water to produce hydrochloric acid. Hydrochloric acid reacts with sodium hydroxide solution. Complete the word equation for the reaction between hydrochloric acid and sodium hydroxide. [1 mark] hydrochloric acid + sodium hydroxide → + water Question 2 continues on the next page 8 *08* IB/M/Jun22/8464/C/1F Do not write outside the box 8 Solutions of hydrochloric acid and sodium hydroxide are reacted and the temperature change is recorded. 0 2 . 3 In the reaction, 3.65 g of hydrogen chloride reacts with 4.00 g of sodium hydroxide. 1.80 g of water is produced. Calculate the mass of the other product. [1 mark] Mass = g 0 2 . 4 Figure 5 shows part of the thermometer used to measure the temperature. Figure 5 What is the temperature reading on the thermometer? [1 mark] Temperature = °C 0 2 . 5 In the reaction, the temperature increases. What type of reaction is happening when the temperature increases? [1 mark] 0 2 . 6 Sodium hydroxide is an alkali. Which two ions are in sodium hydroxide solution? [2 marks] Tick () two boxes. Cl – H+ Na+ O2– OH– 9 *09* Turn over ► IB/M/Jun22/8464/C/1F Do not write outside the 0 3 This question is about structure and bonding. box Figure 6 represents part of the structure of silicon dioxide. Figure 6 0 3 . 1 What type of structure is silicon dioxide? [1 mark] Tick () one box. Giant covalent Ionic lattice Simple molecular 0 3 . 2 Each oxygen atom forms two bonds. What is the number of bonds formed by each silicon atom? Use Figure 6. [1 mark] 10 *10* IB/M/Jun22/8464/C/1F Do not write outside the Figure 7 represents part of a fullerene. box Figure 7 0 3 . 3 Complete the sentence. Choose the answer from the box. [1 mark] hexagons octagons squares triangles The structure of fullerenes is based on . 0 3 . 4 Complete the sentence. Choose the answer from the box. [1 mark] carbon hydrogen oxygen The fullerene molecule shown in Figure 7 is made from atoms of . 11 *11* Turn over ► IB/M/Jun22/8464/C/1F Do not write outside the box 0 3 . 5 What is the fullerene molecule shown in Figure 7 used for? [1 mark] Tick () one box. Electronics Hand warmers Sports injury packs Question 3 continues on the next page 12 *12* IB/M/Jun22/8464/C/1F Do not write outside the Figure 8 box represents part of the structure of a polymer. Figure 8 0 3 . 6 What holds the atoms together in a polymer chain? [1 mark] Tick () one box. Covalent bonds Ionic bonds Metallic bonds 0 3 . 7 Complete the sentence. Choose the answer from the box. [1 mark] atomic intermolecular macromolecular In Figure 8 the polymer chains are held together by forces. 13 *13* Turn over ► IB/M/Jun22/8464/C/1F Do not write outside the box 13 Figure 9 represents part of the structures of: • magnesium metal • a magnesium alloy. Figure 9 0 3 . 8 Calculate the percentage of copper atoms in the alloy. [3 marks] Number of magnesium atoms in the alloy = Number of copper atoms in the alloy = Total number of atoms in the alloy = Percentage of copper atoms in the alloy = % 0 3 . 9 Explain why the magnesium alloy is harder than magnesium metal. Use Figure 9. [3 marks] 14 *14* IB/M/Jun22/8464/C/1F Do not write outside the There are no questions printed on this page box DO NOT WRITE ON THIS PAGE ANSWER IN THE SPACES PROVIDED 15 *15* Turn over ► IB/M/Jun22/8464/C/1F Do not write outside the box 0 4 This question is about elements and compounds. 0 4 . 1 Magnesium and oxygen react to produce magnesium oxide. Balance the equation for the reactio [Show More]
Last updated: 1 year ago
Preview 1 out of 32 pages
Reviews( 0 )
Document information
Connected school, study & course
About the document
Uploaded On
Apr 05, 2023
Number of pages
32
Written in
Additional information
This document has been written for:
Uploaded
Apr 05, 2023
Downloads
0
Views
79












.png)
.png)