Chemistry > QUESTIONS & ANSWERS > 2.1.7 Practice: Reaction Rates. AP Chemistry Sem 2. Points Possible:25. Date: 2/13/2021. With Answer (All)
2.1.7 Practice: Reaction Rates. AP Chemistry Sem 2. Points Possible:25. Date: 2/13/2021. With Answers Provided.
Document Content and Description Below
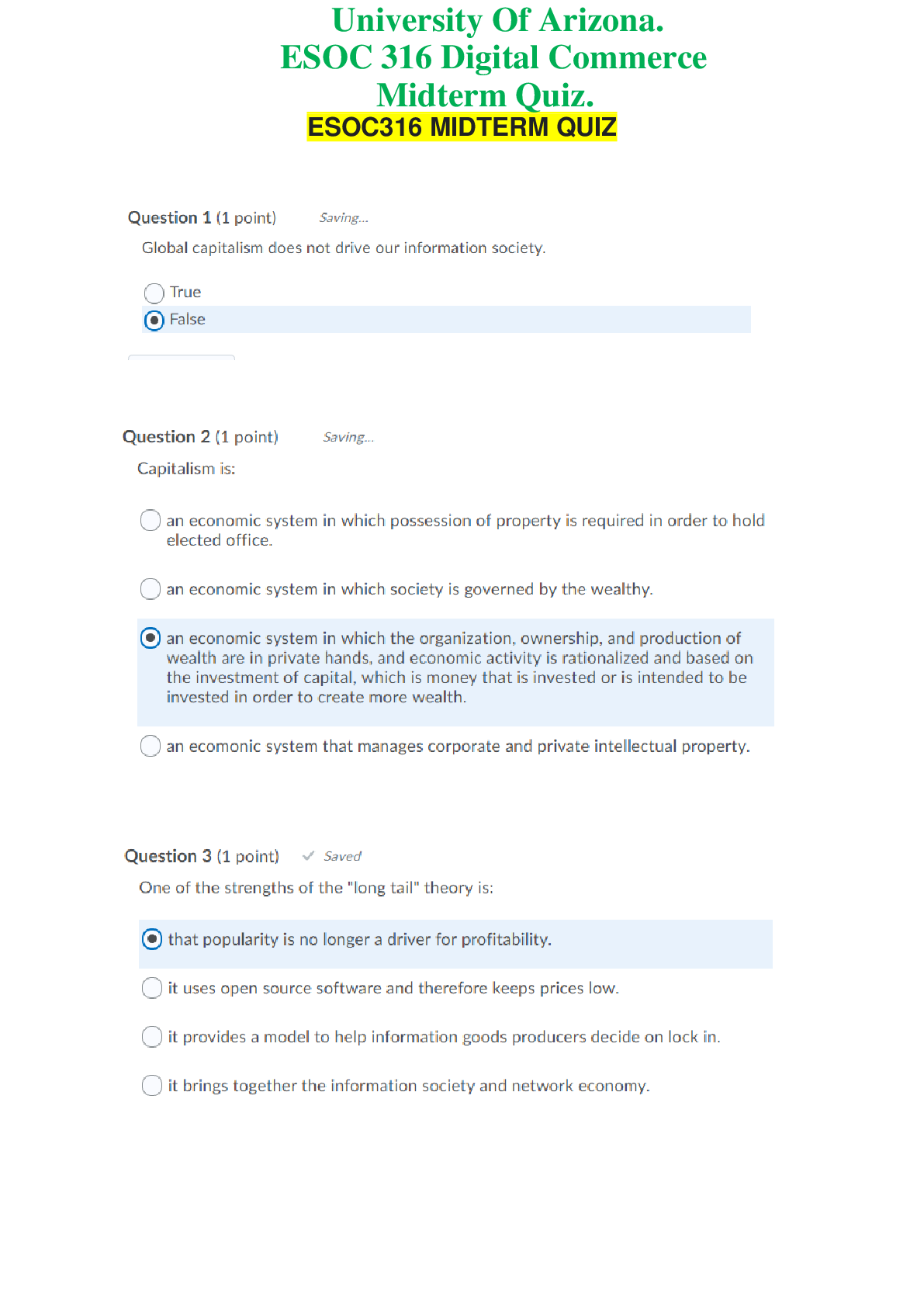
2.1.7 Practice: Reaction Rates AP Chemistry Sem 2 Points Possible:25 Date: 2/13/2021 You will need to use a calculator and a periodic table to complete this activity. 1. Use the given data set ... to determine the rate equation, including the constant, for each of the following reactions. Assume the reaction has the form X + Y → Z. State the overall reaction order for each reaction. a. (7 points) Rate Reaction: Rate = k [X] [Y] 2 Experiment 1 Constant (k): Experiment Initial Concentration of X (mol/L) Initial Concentration of Y (mol/L) Measured Initial Rate (mol/L⋅s) Experiment 2 Constant (k): 0.050 = k [0.1] 2 [0.4] k = 12.5 Experiment 3 Constant (k): 0.075 = k [0.3] 2 [0.2] k = 12.5 Overall Order: 3rd Order b. (7 points) Rate Reaction: Rate = k [X] 2 [Y] 1/2 Experiment Initial Concentration of X (mol/L) Initial Concentration of Y (mol/L) Measured Initial Rate (mol/L⋅s) 1 0.10 0.20 2.57 2 0.10 0.10 1.25 3 0.60 0.10 1.27 Overall Order: 2-and-a-half Order 2. Answer the following questions by connecting the half-life of each first-order reaction to the rate constant. a. The rate constant of a first-order reaction is 2.43 × 10–2 min–1. What is the half-life of the reaction? (2 points) b. A first-order reaction has a rate constant of 0.547 min-1. How long will it take a reactant concentration 0.14 M to decrease to 0.07 M? (2 points) Answer: t1/2 = ln(2)/(0.547) t1/2 = 1.27 minutes Time for reactant concentration to reduce from 0.14 M to 0.07 M: t1/2 = 1.27 minutes c. The half-life of a first-order reaction is 5.47 min. What is the rate constant for the reaction? (2 points) 5.47 = ln(2)/(rate constant) Rate constant = 0.127 min-1 d. A first-order reaction has a half-life of 0.0362 min. What is the rate constant? (2 points) 0.0362 = ln(2)/(rate constant) Rate constant = 19.15 min-1 e. The initial concentration of the reactant X of a first-order decomposition reaction is 0.80 M. After 153 s, the concentration is 0.20 M. What is the rate law for the reaction? (3 points) Rate law: Rate = k [X] Reaction constant: t1/2 = 0.693/k 76.5 = 0.693/k k = 0.0091 [Show More]
Last updated: 1 year ago
Preview 1 out of 5 pages

Buy this document to get the full access instantly
Instant Download Access after purchase
Add to cartInstant download
We Accept:

Reviews( 0 )
$13.00
Document information
Connected school, study & course
About the document
Uploaded On
Apr 20, 2021
Number of pages
5
Written in
Additional information
This document has been written for:
Uploaded
Apr 20, 2021
Downloads
0
Views
106