Chemistry > Lab Report > Molecular Modeling report (All)
Molecular Modeling report
Document Content and Description Below
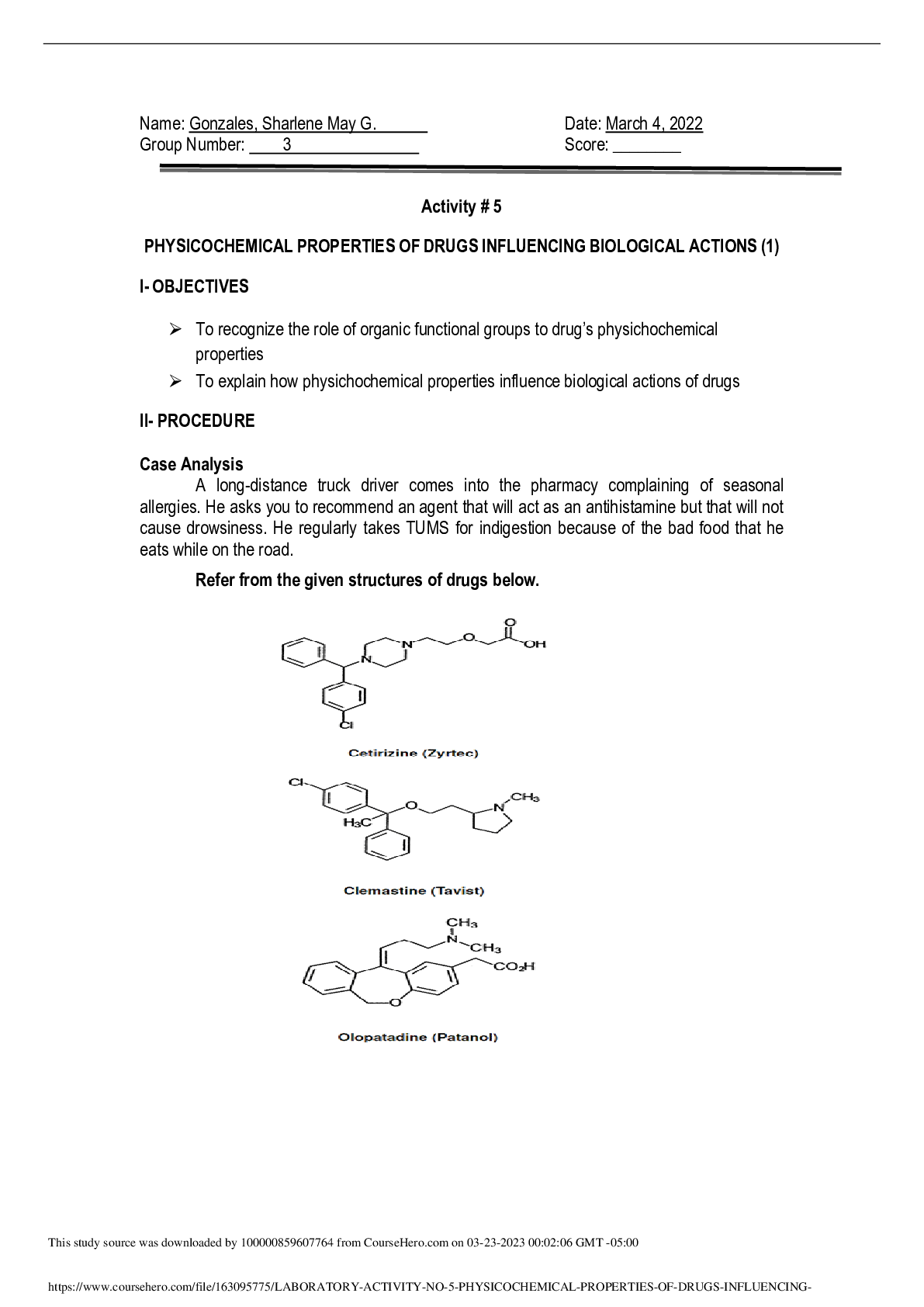
Data Insert photos or scans of each drawn Lewis structure and sketch as described in the lab procedure, include photos of built models, and answer any additional questions. Activity 1 • Explain... why the balls representing fluorine and hydrogen have only one peg, while oxygen has two and carbon has four. Both Fluorine and Hydrogen can only bond with one electron, because Fluorine has 7 valence electrons it can only increase to 8 for stability, and Hydrogen only has one valence electron and one bonding domain meaning it can bond but only to one molecule. Meanwhile, because Oxygen has 6 valence electrons it can only take up two more to satisfy the octet rule, and Carbon has four because Carbon has 4 valence electrons meaning it has 4 open domains for a bonding to occur. To add to this, in terms of pegs, Oxygen can make double bonds, and Carbon in incredibly rare cases can make quadruple bonds, while Fluorine and Hydrogen will only make single bonds Activity 2 [Show More]
Last updated: 1 year ago
Preview 1 out of 13 pages

Also available in bundle (1)

Molecular Geometry
Molecular Geometry
By Muchiri 3 years ago
$11
2
Reviews( 0 )
Document information
Connected school, study & course
About the document
Uploaded On
Apr 20, 2021
Number of pages
13
Written in
Additional information
This document has been written for:
Uploaded
Apr 20, 2021
Downloads
0
Views
51




.png)







.png)
.png)





 (1).png)