Chemistry > Lab Experiment > Wellesley College REL 216 Edited - Concentration Lab - Casey McGee-VERIFIED A+ (All)
Wellesley College REL 216 Edited - Concentration Lab - Casey McGee-VERIFIED A+
Document Content and Description Below
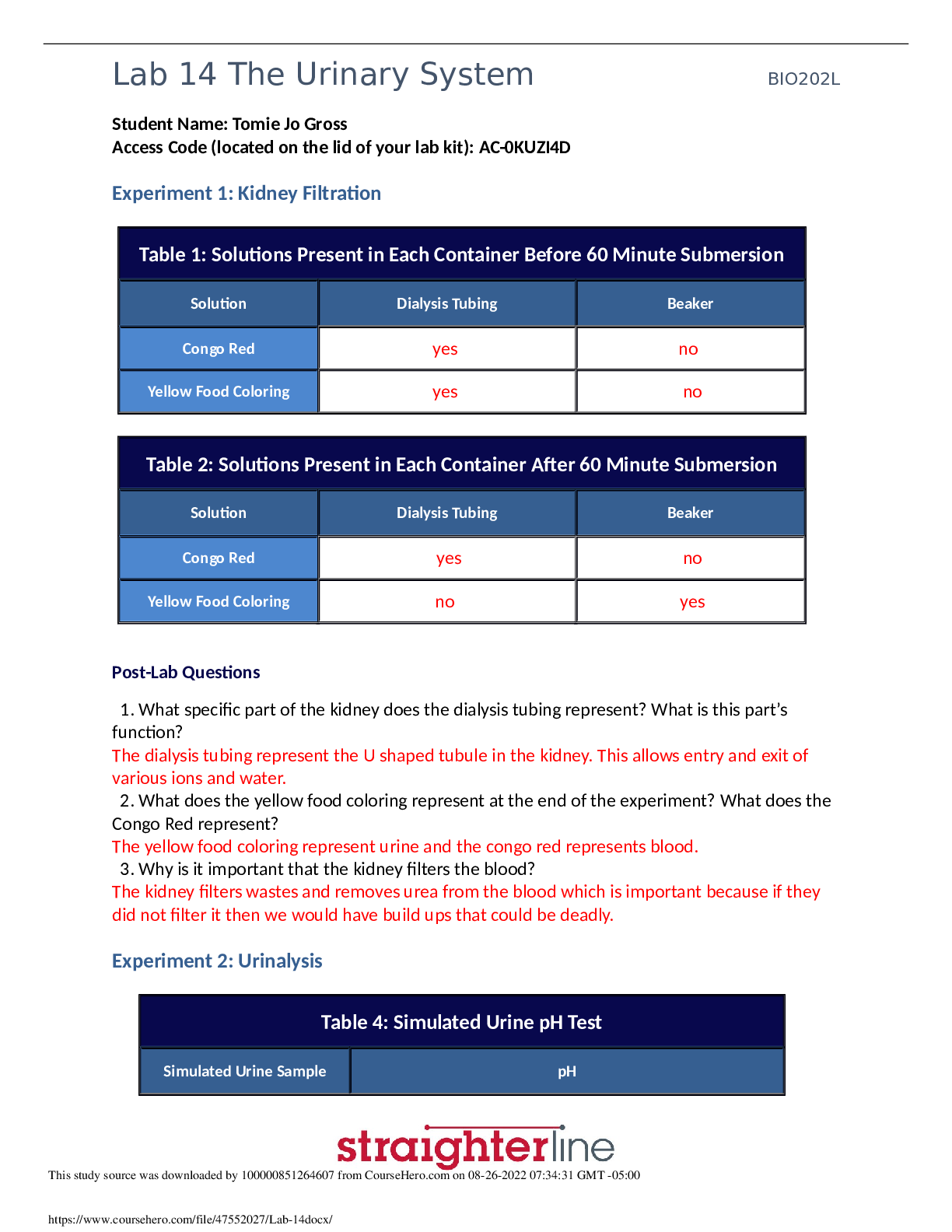
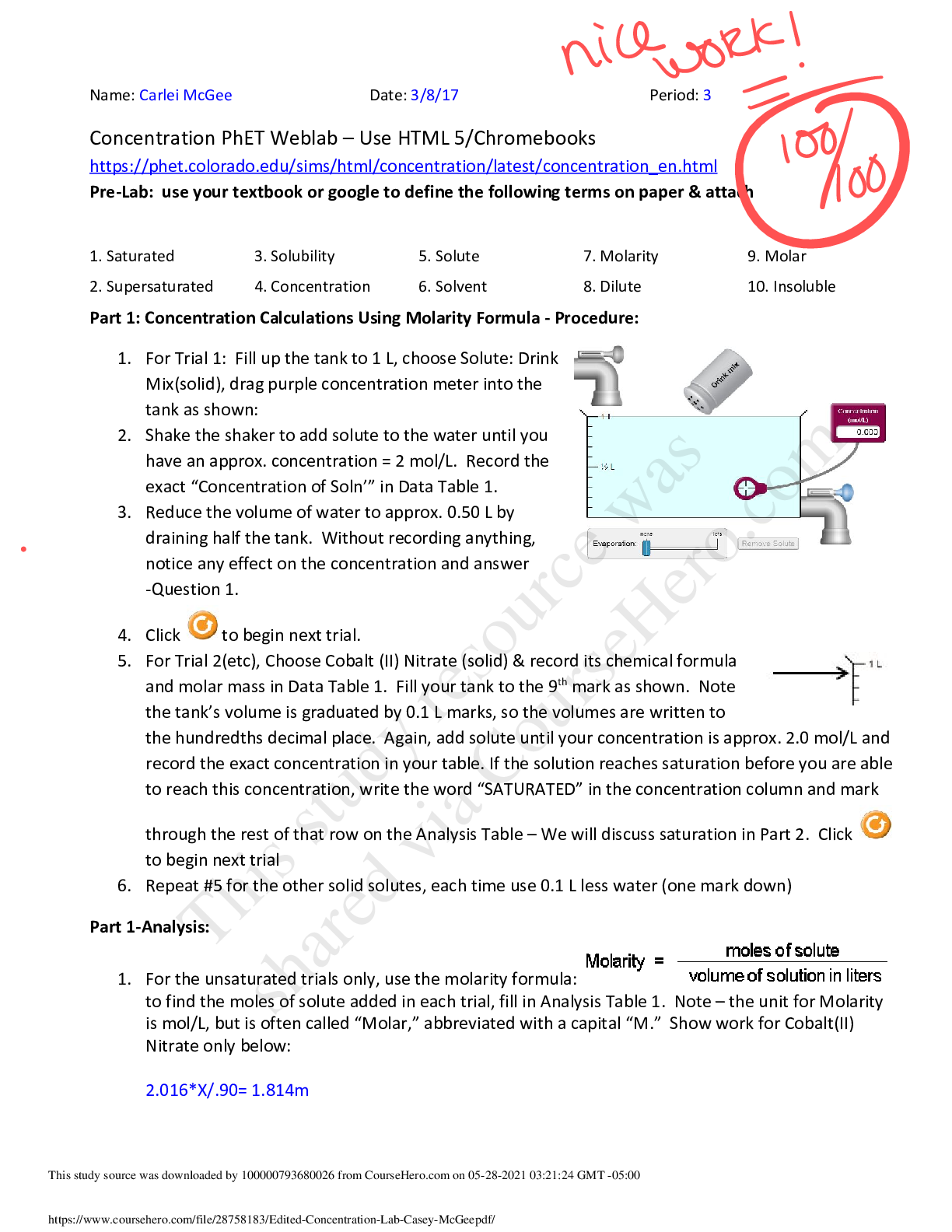
Concentration PhET Weblab – Use HTML 5/Chromebooks https://phet.colorado.edu/sims/html/concentration/latest/concentration_en.html Pre-Lab: use your textbook or google to define the following terms... on paper & attach 1. Saturated 2. Supersaturated 3. Solubility 4. Concentration 5. Solute 6. Solvent 7. Molarity 8. Dilute 9. Molar 10. Insoluble Part 1: Concentration Calculations Using Molarity Formula - Procedure: 1. For Trial 1: Fill up the tank to 1 L, choose Solute: Drink Mix(solid), drag purple concentration meter into the tank as shown: 2. Shake the shaker to add solute to the water until you have an approx. concentration = 2 mol/L. Record the exact “Concentration of Soln’” in Data Table 1. 3. Reduce the volume of water to approx. 0.50 L by draining half the tank. Without recording anything, notice any effect on the concentration and answer -Question 1. 4. Click to begin next trial. 5. For Trial 2(etc), Choose Cobalt (II) Nitrate (solid) & record its chemical formula and molar mass in Data Table 1. Fill your tank to the 9th mark as shown. Note the tank’s volume is graduated by 0.1 L marks, so the volumes are written to the hundredths decimal place. Again, add solute until your concentration is approx. 2.0 mol/L and record the exact concentration in your table. If the solution reaches saturation before you are able to reach this concentration, write the word “SATURATED” in the concentration column and mark through the rest of that row on the Analysis Table – We will discuss saturation in Part 2. Click to begin next trial 6. Repeat #5 for the other solid solutes, each time use 0.1 L less water (one mark down) Part 1-Analysis: 1. For the unsaturated trials only, use the molarity formula: to find the moles of solute added in each trial, fill in Analysis Table 1. Note – the unit for Molarity is mol/L, but is often called “Molar,” abbreviated with a capital “M.” Show work for Cobalt(II) Nitrate only below: 2.016*X/.90= 1.814m [Show More]
Last updated: 1 year ago
Preview 1 out of 5 pages
Reviews( 0 )
Document information
Connected school, study & course
About the document
Uploaded On
May 28, 2021
Number of pages
5
Written in
Additional information
This document has been written for:
Uploaded
May 28, 2021
Downloads
0
Views
52













.png)


.png)










.png)