Chemistry > QUESTIONS & ANSWERS > CHEM 120 Week 8 Final Exam graded A (All)
CHEM 120 Week 8 Final Exam graded A
Document Content and Description Below
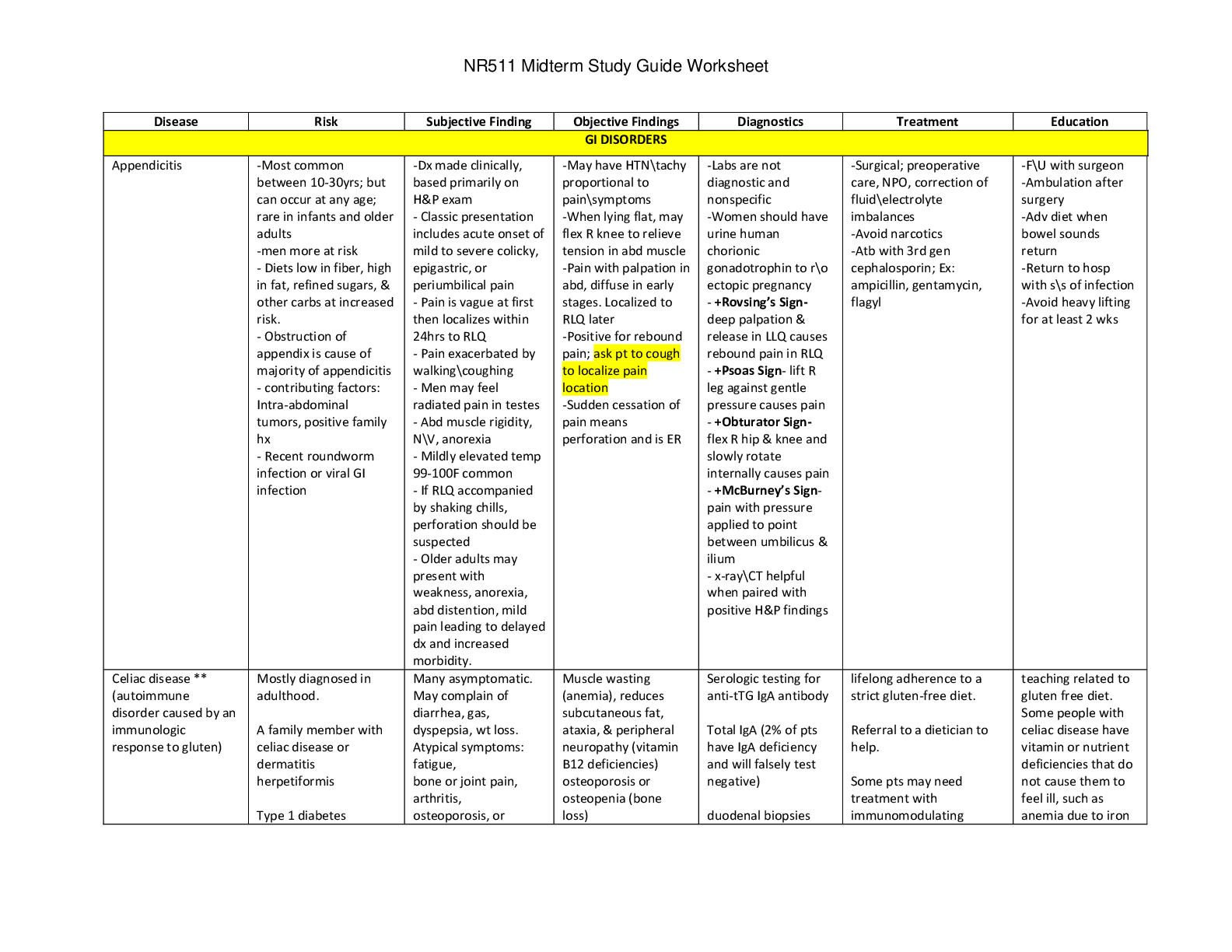
CHEM 120 Week 8 Final Exam 1.(TCO 6) A gas at a temperature of 95 degrees C occupies a volume of 165 mL. Assuming constant pressure, determine the volume at 25 degrees C. Show your work. (Points :... 5) Using Charles’ Law, (V1/T1) = (V2/T2). First, convert temperature to KELVIN (T1 = t1 +273) Thus, T1 = 95 + 273 = 368. We have V1 (165 mL) & T2 = (25 + 273) = 298. V2 = (V1*T2)/T1 = (165 mL*298)/368 = 133.6 mL. 0 1644446457 Short 16 2.(TCO 6) A sample of helium gas occupies 1021 mL at 719 mmHg. For a gas sample at constant temperature, determine the volume of helium at 745 mmHg. Show your work. (Points : 5) 1021mL * 719 mm/745 mmHg = 985.36mL =985mL Using Boyle’s law, P1V1 = P2V2. We have V1 (1021 mL), P1 (719 mmHg) and P2 (745 mmHg). 0 1644446459 Short 19 2.(TCO 12) If one strand of a DNA double helix has the sequence T T A G C G A C G C, what is the sequence of the other DNA strand? (Points : 10) A A T C G C T G C G 1. (TCO 8) 35.0 mL of 0.25 M NaOH is neutralized by 23.6 mL of an HCl solution. The molarity of the HCl solution is (show your work): (Points : 5) 2. (TCO 1) How many mL are in 3.5 pints? Show your work. (Points : 5) 0.25 M = moles NaOH / 0.035 L moles NaOH = 0.00875 moles NaOH4.(TCO 3) What is the name of the following compound: Zn3P2? (Points : 5) 3.5 pints* 473.176mL = 1656.116mL3 3. (TCO 3) What is the name of the following compound: AgNO3? (Points : 5) 4. (TCO 6) Calculate the pressure, in atmospheres, of 2.78 mol CO(g) in a 4.25 L tank at 51 degrees C. (Points : 5) 1. (TCO 7) (a, 5 pts) Given that the molar mass of H3PO4 is 97.994 grams, determine the number of grams of H3PO4 needed to prepare 0.75L of a 0.25M H3PO4 solution. Show your work. (b, 5 pts) What volume, in Liters, of a 0.25 M H3PO4 solution can be prepared by diluting 50 mL of a 2.5M H3PO4 solution? Show your work. (Points : 10) Using the molar mass given, convert this amount to grams. mass = 0.1875 mol * (97.994 g/mol) = 18.37 grams H3PO4 b. M1*V1 = M2*V2 w here: M1 = 0.25; V1 = ??; M2 = 2.5; V2 = 50 mL = 0.050 L Solvig for V1: 0.25 * V1 = 2.5 * 0.050 V1 = 0.50 L 2. First convert the given mass of NaOH to volume (in mL) using the density of NaO Volume = 43 g * (1 mL/2.13 g) = 20.19 mL H Volume % = (volume of solute / volume of solution) * 100% Volume % = (20.19 mL/120 mL) * 100% = 16.8 % b. Volume % = volume of NaOH/ total volume 0.10 = 20.19 mL/total volume Solving for total volume yields: [Show More]
Last updated: 1 year ago
Preview 1 out of 23 pages

Reviews( 0 )
Document information
Connected school, study & course
About the document
Uploaded On
Jun 25, 2021
Number of pages
23
Written in
Additional information
This document has been written for:
Uploaded
Jun 25, 2021
Downloads
0
Views
45