BioChemistry > STUDY GUIDE > BIOL 3362 - Exam 4 Study Guide (Biochemistry, Proteins, Fatty Acids, Fatty acid metabolism, fatty ac (All)
BIOL 3362 - Exam 4 Study Guide (Biochemistry, Proteins, Fatty Acids, Fatty acid metabolism, fatty acid synthesis).
Document Content and Description Below
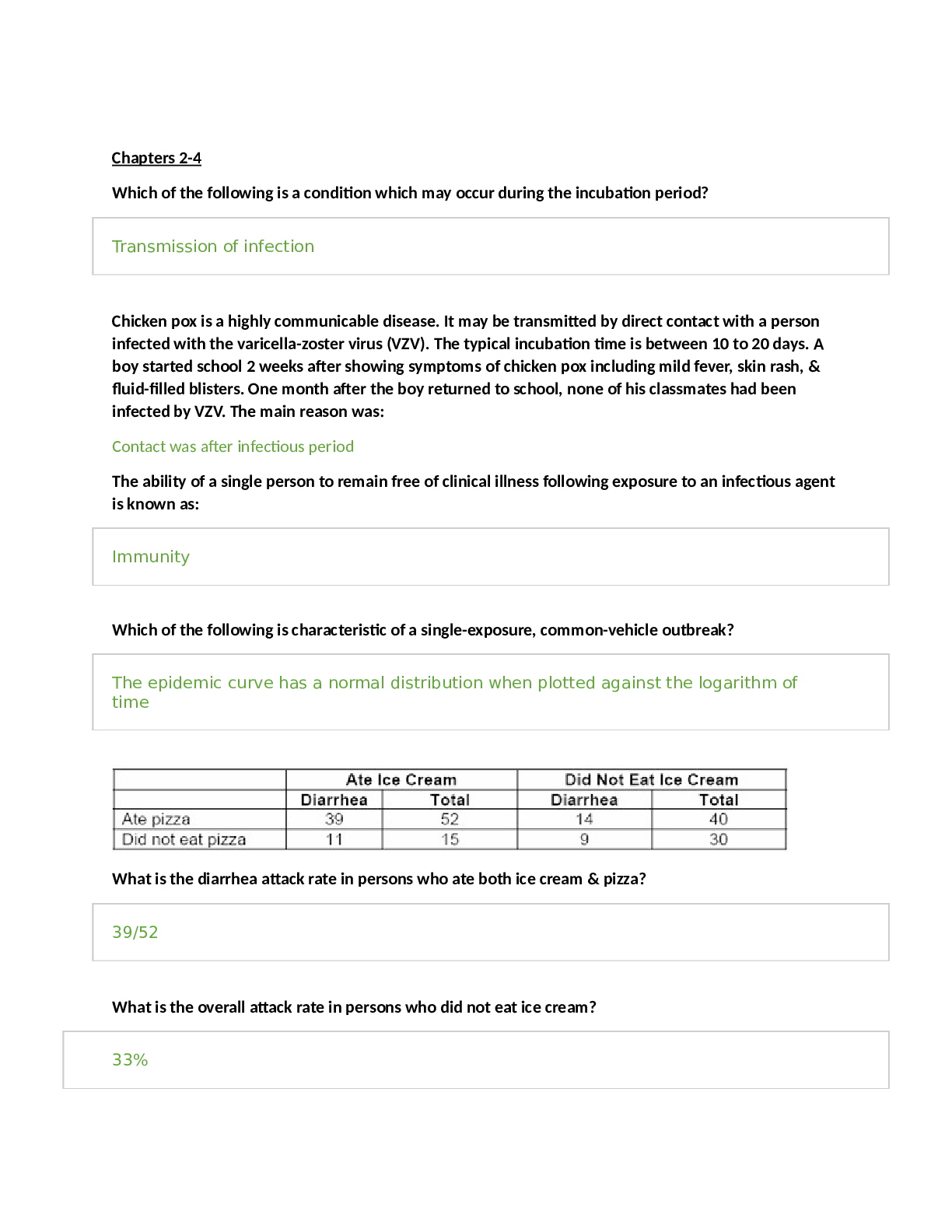
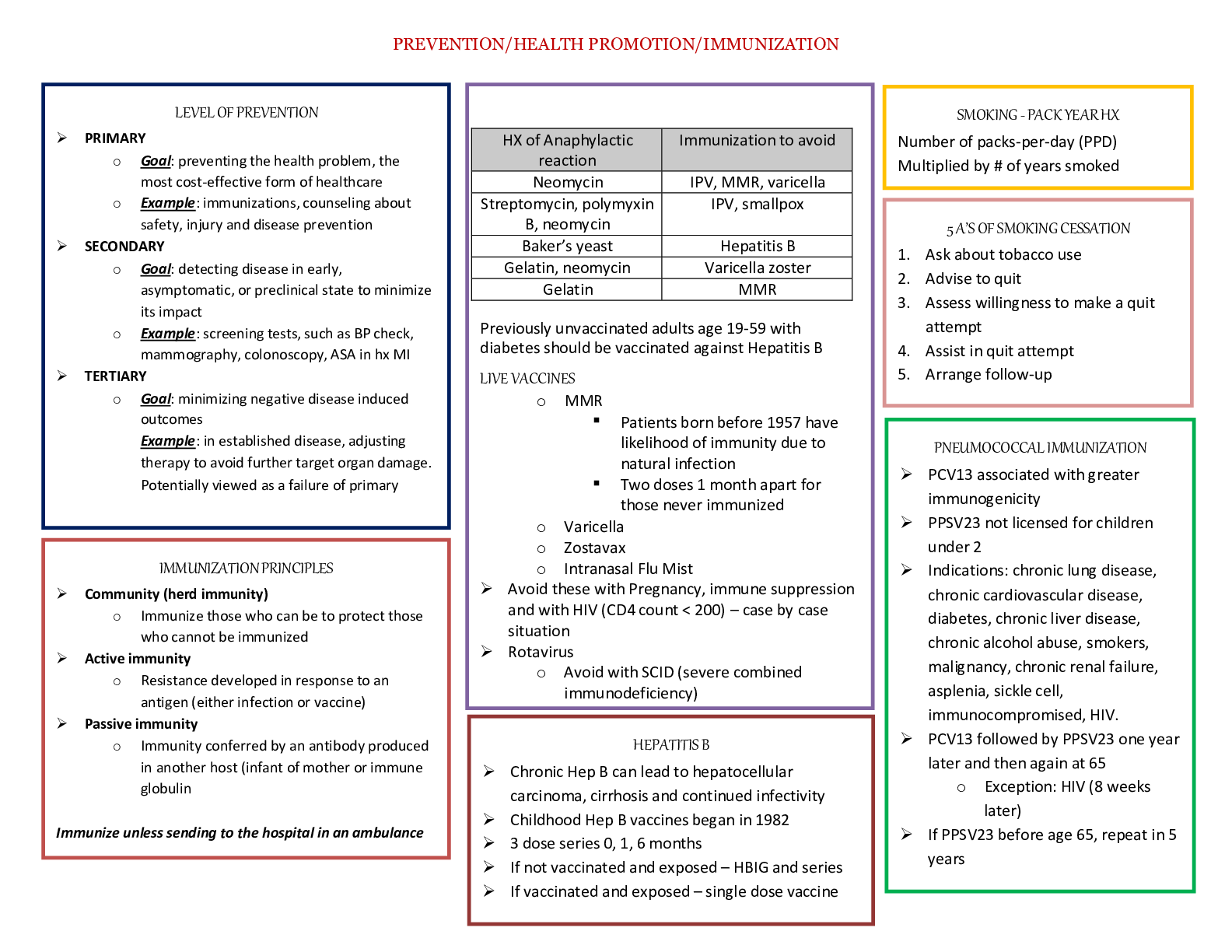
Biochemistry II Exam 4 Chapter 24 Lipid Biosynthesis What is the difference between fatty acid synthesis and fatty acid degradation? ● Use of CoA and acyl protetin: Intermediates in synthesi... s are linked to -SH groups of acyl carrier proteins like phosphopantetheine and cysteine(as compared to -SH groups of CoA in breakdown) ● Cellular compartment: Synthesis in cytosol; breakdown in mitochondrial matrix ● Enzymes of fatty acid synthesis are components of one long polypeptide chain, the fatty acid synthase, whereas no similar association exists for the degradative enzymes. ● Use of NADPH and NADH: Biosynthesis uses NADPH/NADP+, breakdown uses NADH/NAD + ● Addition and removal of water: Addition of water degrades fatty acids while removing water synthesizes fatty acids. Where and how NADPH is produced for fatty acid synthesis? ● NADPH can be produced in the pentose phosphate pathway as well as by malic enzyme, which converts malate to pyruvate ● Reducing equivalents derived from glycolysis in the form of NADH can be transformed into NADPH by malate dehydrogenase and malic enzyme ● When malate is oxidized, one NADPH is produced for every acetyl-CoA. Conversion of 8 acetyl- CoA units to one palmitate would then be accompanied by production of 8 NADPH. How is acetate activated for fatty acid synthesis? Acetate units are activated for transfer by malonyl-CoA at the expense of ATP. The carboxylation of acetyl-CoA is a committed step and is catalyzed by acetyl-CoA carboxylase. The carbon dioxide is lost later during condensation with the growing fatty acid. The spontaneous decarboxylation drives the condensation reaction and drive chain growth. What is the longest product of fatty acid synthesis? The chain grows to 16 carbons, which makes up a fatty acid called palmitic acid. Other enzymes add double bonds and additional carbon units to the chain. What are the three main sources for acetyl-CoA for fatty acid synthesis? 1. Amino acid degradation produces cytosolic acetyl-CoA 2. Fatty acid oxidation produces mitochondrial acetyl-CoA 3. Glycolysis yields cytosolic pyruvate which in converted to acetyl-CoA in mitochondria What is the citrate-malate-pyruvate shuttle? The citrate-malate-pyruvate shuttle provides cytosolic acetate units and reducing equivalents for fatty acid synthesis. The shuttle collects carbon substrates, primarily from glycolysis but also from fatty acid oxidation and amino acid catabolism. (picture on slide 10 shows it is not part of citrate-malate-pyruvate shuttle) The notes do mention that amino acid catabolism does provide carbons to the shuttle. Amino acid catabolism causes amino acids to be broken down into acetyl-CoA, which does not contribute to the shuttle. However, aspartate and asparagine is broken down into oxaloacetate. Most of the reducing equivalents are glycolytic in origin. In summary, the shuttle is a source for acetate units to build fatty acids. What is the function of malic enzyme? Malic enzyme converts malate into pyruvate by decarboxylation, which will also convert NADP+ to NADPH. Oxaloacetate is converted to malate through malate dehydrogenase first. What is the function of biotin in fatty acid synthesis? The biotin prosthetic group of acetyl-CoA carboxylase (ACC) is covalently linked to the ε-amino group of an actice-site lysine. Acetyl-CoA carboxylase is biotin-dependent. The long, flexible biotin-lysine chain enables the activated carboxyl group to be carried between the biotin carboxylase and the carboxyltransferase. This helps with transfering activated CO2 to acetyl-CoA. Know the connections between glycolytic, fatty acid degradation and synthesis pathways. Know the key intermediates and related enzymes in these connections. ● Glucose is converted to acetyl-CoA by glycolysis and combines with oxaloacetate via citrate synthase to form citrate in the mitochondria. ● The citrate is transported to the cytosol, breaks up into acetyl-CoA and oxaloacetate via ATP- citrate lyase. Some of the acetyl-CoA is carboxylated to malonyl-CoA by ACC and then proceeds to fatty acid synthesis. The oxaloacetate becomes malate through malate dehydrogenase and can head back into the matrix to become oxaloacetate again or be further decarboxylated into pyruvate through malic enzyme. ● Synthesis is done by fatty acid synthase, performing the condensation of acetyl-CoA and malonyl-CoA to produce palmitate. ● Finally, fatty acid degradation produces fatty acyl-CoA, which converts to fatty acyl-carnitine, oxidizes in the mitochondrial matrix and becomes acetyl-CoA Look at slide 10 and slide 73 figure. How is malonyl-CoA formed? Carboxylation of acetyl-CoA, catalyzed by acetyl-CoA carboxylase. ATP and bicarbonate is needed as well. How is Acetyl-CoA carboxylase regulated? Citrate allosterically activates acetyl-CoA carboxylase (ACC). Citrate is high when there is adequate acetyl-CoA entering Krebs Cycle. Excess acetyl-CoA is then converted via malonyl-CoA to fatty acids for storage. ACC is allosterically inhibited by palmitoyl-CoA and is allosterically not stimulated by ATP. Unphosphorylated ACC binds citrate with high affinity and thus is active. Phosphorylating ACC by protein kinase A attached means ACC does not any affinity for citrate and you need high levels of citrate to activate ACC and is sensitive to levels of fatty acids to inhibit ACC. Dephosphorylating ACC means that it is a high affinity for citrate and is more sensitive to citrate, so it is more prone to become active. What is the difference in fatty acid synthase between bacteria, plants, animal, and fungi? Enzymes of fatty acid synthesis in fatty acid synthase are components of one long polypeptide chain in animals. Plants and bacteria employ separate enzymes to carry out the biosynthetic reactions. Mammals - fatty acid synthase I (FAS I), which is homodimeric, which consists of 270 kD polypeptides which contain all reaction centers required to produce a fatty acid. The enzymes are all component of one long polypeptide chain. Plants and most bacteria - Here the enzymes are independent from each other, and collection of enzymes are called FAS II. Yeast and fungi- activities of fatty acid synthase are distributed on two multifunctional peptide chain. What type of reaction is used to convert acetyl-CoA to malonyl-CoA? Carboxlyation of acetyl-CoA and as well as NADP+ reducing to NADPH (not part of this ACC catalyzed reaction). Correction:NADPH is produced by pentose phosphate pathway and malic enzyme, but not ACC reaction. ATP is definitely hydrolyzed, though. How do unsaturation and further elongation of fatty acids occur in eukaryotes? Longer chains are made through special elongation reactions, which occur both in the mitochondria and the surface of the ER (I thought it only occurs in the mitochondria). The textbook mentions that elongation occurs at the ER. The ER reactions are similar to the addition of two-carbon units at the carboxyl end of the chain by means of oxidative decarboxylation involving malonyl-CoA. This decarboxylation provides the thermodynamic driving force for the condensation reaction. The mitochondrial reactions involve addition of acetyl units. These reactions are essentially a reversal of fatty acid oxidation, with the exception that NADPH is utilized in the saturation of the double bond, instead of FADH2. Elongation of fatty acids in mitochondria is initiated by the thiolase reaction. The b-ketoacyl intermediate thus formed undergoes the same three reactions (in reverse order) that are the basis of b- oxidation of fatty acids. Reduction of the b-keto group is followed by dehydration to form a double bond. Reduction of the double bond yields a fatty acyl-CoA that is elongated by two carbons. Note that the reducing coenzyme for the second step is NADH, whereas the reductant for the fourth step is NADPH. For unsaturation, eukaryotes have adopted an O2-dependent reaction. The addition of double bonds to fatty acids in eukaryotes does not occur until the fatty acyl chain has reached its full length. Dehydrogenation occurs in the middle of the chain, despite the absence of any useful functional group on the chain to facilitate activation. Double bonds are introduced into the growing fatty acid chain in E. coli by specific dehydrases. Palmitoleoyl-ACP is synthesized by a sequence of reactions involving four rounds of chain elongation, followed by double bond insertion by b-hydroxydecanoyl thioester dehydrase and three additional elongation steps. The double bond is cis. Another elongation cycle produces cis-vaccenic acid. The conversion of stearoyl-CoA to oleoyl-CoA in eukaryotes is catalyzed by stearoyl-CoA desaturase in a reaction sequence that also involves cytochrome b5 and cytochrome b5 reductase. Two electrons are passed from NADH through the chain of reactions as shown, and two electrons are also derived from the fatty acyl substrate. What are the factors that regulate fatty acid synthesis and degradation? Fatty acid synthesis occurs in the cytosol. Acetyl-CoA generated in mitochondria is transported to the cytosol via a shuttle mechanism involving citrate. Palmitate, a 16-C saturated fatty acid, is the final product of the Fatty Acid Synthase reactions. Summary (ignoring H+ & water): acetyl-CoA + 7 malonyl-CoA + 14 NADPH --> palmitate + 7 CO2 + 14 NADP+ + 8 CoA Accounting for ATP-dependent synthesis of malonate: 8 acetyl-CoA + 14 NADPH + 7 ATP --> palmitate + 14 NADP+ + 8 CoA + 7 ADP + 7 Pi •Regulatory control of fatty acid metabolism is an interplay of allosteric modifiers, phosphorylation and dephosphorylation cycles and hormones .Malonyl-CoA blocks the carnitine acyltransferase and thus inhibits beta-oxidation. •Citrate activates acetyl-CoA carboxylase (ACC) •Fatty acyl-CoAs inhibit ACC •Hormones regulate ACC •Glucagon activates lipases/inhibits ACC •Insulin inhibits lipases/activates ACC Hormonal signals regulate fatty acid synthesis, primarily through actions on acetyl-CoA carboxylase, with additional effects on triacylglycerol lipase. Regulation of fatty acid synthesis and fatty acid oxidation are coupled as shown. Malonyl-CoA, produced during fatty acid synthesis, inhibits the uptake of fatty acylcarnitine (and thus fatty acid oxidation) by mitochondria. When fatty acyl-CoA levels rise, fatty acid synthesis is inhibited and fatty acid oxidation activity increases. Rising citrate levels (which reflect an abundance of acetyl-CoA) similarly signal the initiation of fatty acid synthesis. Hormonal signals regulate fatty acid synthesis, primarily through actions on acetyl-CoA carboxylase. Availability of fatty acids also depends upon hormonal activation of triacylglycerol lipase. Many of the enzymes that act to phosphorylate ACC are controlled by hormonal signals. Glucagon binding to membrane receptors activates an intracellular cascade involving activation of adenylyl cyclase. cAMP produced by the cyclase activates a protein kinase, which then phosphorylates ACC. Unless citrate levels are high, phosphorylation of ACC causes inhibition of fatty acid synthesis. Phosphatases reverses this inhibition via dephosphorylation. Glucagon also activates triaglycerol lipases, which hydrolyze triacylglycerols, releasing fatty acids for beta-oxidation (use of energy when glucose is low) Both the inactivation of acetyl-CoA carboxylase and the activation of triacylglycerol lipase are counteracted by insulin, whose receptor acts t stimulate a phosphodiesterase that converts cAMP to AMP. Insulin will promote dephosphorylation (no cAMP for protein kinases) of ACC and causes initiation of fatty acid synthesis, activating ACC. Insulin also inhibit lipases since you don't need the energy from triacylglycerols if there is plenty of glucose. How do the nutritional state and cellular needs regulate fatty acid metabolism? Excess carbon consumed as carbs are converted to fat by FAS in liver and adipose tissue. They are stored as triglycerides. FAS is responsible primarily for energy storage by converting excess carbohydrate to fatty acids that are then esterified to store triacyglycerols. What is the hormonal response to decrease in blood sugar? Glucagon will raise blood sugar if it gets too low. This will also activate lipases (need the energy from fat) and inhibits ACC (synthesizing fatty acids require energy, and there is not enough of that), so lipid breakdown occurs and lipid biosynthesis is inhibited. What is the hormonal response to increase in blood sugar? Insulin will lower blood sugar if it gets too high. This will do the opposite effects of glucagon, so it inactivates lipases (don't need the energy from fat) and promotes ACC (have the energy to synthesize fatty acids), so lipid breakdown does not occur and lipid biosynthesis occurs. What are the main compounds utilized in the synthesis of triacylglycerols and glycerophospholipids? Dihydroxyacetone phosphate (DHAP) or glycerol forms phosphatidic acid, which is the precursor for all other glycerolipids in eukaryotes. Phosphatidic acid is made into either diacylglycerol (DAG) or CDP- DAG. CDP-DAG provides the phosphatidyl moiety of PI, PG, CL. Slide 88 on chapter 24. PE and PC are formed by reaction between DAG with CDP-E, and CDP-C. (PS is derived from PE, and thus should come from DAG). This is verified. For triacylglycerols, they are formed from 2-monoacylglycerol and basically an monoacylglycerol transferase will transfer acyl-CoAs to the 2-MAG to make DAG. DAG will then become a triacylglycerol though another acyl transfer. Eukaryotic acyltransferases use only fatty acyl-CoA molecules as substrates. What is the role of CTP in phospholipid synthesis? CTP drives formation of CDP complexes. CTP plays an essential role in the synthesis of all membrane phospholipids because it is the direct precursor of the activated, energy-rich phospholipid pathway intermediates CDP-diacylglycerol, CDP-choline, CDP-ethanolamine. Basically. CTP can become CDP- intermediates and then you get PS, PE, PC and PI. What is the precursor to the synthesis of complex lipids? What are the major lipids synthesized by using this precursor? Phosphatidic acid is the precursor for all other glycerolipids, and is converted directly either to DAG or to CDP-DAG from which all other glycerophospholipids are derived. Triacylglycerols, phospholipids such as PI, PG, CL, PS, PE, PC are major lipids synthesized by this precursor. Condensation of Serine and palmitoyl – CoA produce ceramide which can then be used to form sphingolipid What is the precursor to plasmalogens? Dihydroxyacetone phosphate (DHAP) is the precursor for plasmalogen biosynthesis. You also need a long chain alcohol, NADH, NADPH, and O2, fatty acyl-CoA, and CDP-ethanolamine. Slide 94 for the synthesis of plasmalogens. Why are phospholipases important to cell signaling? Prostaglandins are found in most tissues and organs. They are produced by almost all nucleated cells. They are autocrine and paracrine lipid mediators that act upon platelets, endothelium, uterine and mast cells. They are synthesized in the cell from the essential fatty acids (EFAs). An intermediate, arachidonic acid, is created from phospholipids via phospholipase-A2, then brought to either the cyclooxygenase pathway or the lipoxygenase pathway to form either prostaglandin and thromboxane or leukotriene respectively. Phospholipases release arachdonic acid from phospholipids and DAG, and arachidonic acid is also the precursor to eicosanoids. Eicosanoids are signaling molecules and exert control over many bodily system, mainly in inflammation or immunity, and as messengers in the central nervous system. How are sugars added in glycosphingolipid synthesis? The biosynthesis of monoglycosylceramides requires a direct transfer of the carbohydrate moiety from a sugar-nucleotide, such as uridine 5-diphosphate(UDP)-galactose, or UDP-glucose to the ceramide unit. The glycosyl-transferase catalyzed reaction results in an inversion of the glycosidic bond stereochemistry, changing from α →β. Synthesis of galactosylceramide, and glucosylceramide occurs on the lumenal surface of the endoplasmic reticulum, and on the cytosolic side of the early Golgi membranes respectively. What are eicosanoids? When and how are they produced? What is the precursor? What is/are the main reactions? Eicosanoids are the major products derived from the cellular metabolism of arachidonic acid by the enzymes cyclooxygenase and lipoxygenase. They are the breakdown products of phospholipids. The eicosanoids comprise of prostaglandins, thromboxanes, prostacyclins, leucotrienes, lipoxins. The production of eicosanoids is initiated by the release of C20-polyunsaturated fatty acids such as arachidonic acid, from phospholipids or DAG. Cytosolic phospholipase A2 (cPLA2) catalyzes this reaction. Eicosanoids are produced in the membrane and the cytosol. How they are produced: 1). Arachidonic acid is first liberated from membrane glycerophospholipids by the actions of the phospholipase A2 (PLA2) family of enzymes. 2). Liberated fatty acids are then oxygenated either through prostaglandin synthase pathway, including cyclooxygenase (COX) activity, or the lipoxygenase-depednet pathway. 3). The cyclooxygenase (COX) enzymes then catalyze the biosynthesis of the bicyclic endoperoxide intermediate PGG2, followed by reduction to PGH2. 4). Conversion to prostaglandins (PGs), prostacyclins, thromboxanse (TXs), and leukotrienes. Specifically, PGH2 is subsequently converted to one of several structurally related prostaglandins (PGs), including PGE2, PGD2, PGF2 , PGI2 and thromboxane A2 (TxA2), by the activity of specific PG synthases. What is/are the drug targets for prostaglandin synthesis? Prostaglandin endoperoxide H synthase possesses two activities: cyclooxygenase (COX) and a glutathione-dependent peroxidase (POX). The mechanism of the reaction begins with hydrogen atom abstraction by a tyrosine radical on the enzyme, followed by rearrangement to cyclize and incorporate two oxygen molecules. Reduction of the peroxide at C-15 completes the reaction. COX is the site of action of aspirin and other analgesic agents. How do the cyclooxygenase inhibitors work? Traditional non-steroidal anti-inflammatory drugs (NSAIDs) inhibit the activity of both COX-1 and COX-2, whereas the recently developed coxib family of NSAIDs selectively inhibit COX-2. COX-1 is responsible for 'housekeeping' PG biosynthesis and is constitutively produced in most tissues in the body. COX-2, on the other hand, is not normally produced in most tissues but is induced by a wide spectrum of growth factors and pro-inflammatory cytokines in specific pathophysiological conditions. On the medical side, COX-2 inhibitors are nonsteroidal anti-inflammatory drugs, and are prescribed to treat arthritis and muscle pain. Numerous studies have found increased risk of heart attack, stroke, and blood clots in patients using COX-2 inhibitors. This class of drugs was intended to treat pain with decreased gastrointestinal side effects associated with the older NSAIDs such as ibuprofen and naproxen. Cyclooxygenase inhibitors are applied in order to reduce inflammation, to relieve from pain, or to prevent atherothrombotic complications in cardiovascular disease. Cyclooxygenases represent a major target of pharmaceutical therapy. COX-2 inhibitors work by blocking the COX-2 inhibitors work by blocking the COX-2 enzyme, whereas older NSAIDs block both COX-1 and COX-2 enzymes. Aspirin inhibits COX activity of endoperoxide synthase (makes prostaglandins) via acetylation of Ser-530. COX-2 inhibitors selectively block inflammation, have less potential for stomach and kidney problems than aspirin and COX-1 inhibitors. What is the committed step in cholesterol biosynthesis? Catalyzed by HMG-CoA reductase, the committed step occurs when HMG-CoA is reduced to 3R- mevalonate. What compounds are used to form mevalorate? 1. Two acetyl-CoA's combine to make acetoacetyl-CoA and that is added to another acetyl-CoA to make HMG-CoA. 2. HMG-CoA, NADPH (two of them in reductive succession), CoASH leaves, making the primary alcohol 3R- mevalonate. Learn the regulation of HMG-CoA reductase. There are three ways to regulate HMG-CoA reductase: 1. Phosphorylation by cAMP-dependent protein kinases inactivates the reductase. This inactivation can be reversed by two phosphatases. 2. Degradation via half life. HMG-CoA reductase as a half-life of only 3 hours, and the half-life itself depends on cholesterol levels. High [cholesterol] levels means a short half-life for HMG-CoA reductase. 3. Gene expression. Cholesterol levels control the amount of mRNA. If [cholesterol] is high, levels of mRNA coding for the reductase is reduced. If [cholesterol] is low, levels of mRNA coding increase. What is the most common drug target for cholesterol metabolism? HMG-CoA reductase is site of action of cholesterol-lowering drug; this is the rate-limiting committed step in cholesterol biosynthesis so it makes sense for this to be regulated. How do the statin drugs work? Inhibition of 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase by statins inhibits cholesterol synthesis and isoprenoid production. Mevinolin and similar drugs are cholesterol lowering drugs that act as transition state analogs, binding like transition states to the HMG-CoA reductase. Lovastatin blocks HMG-CoA reductase and is hydrolyzed to become mevinolinic acid which is a competitove inhibitor of the reductase. Normally in mammalian cells this enzyme is suppressed by cholesterol derived from the internalization and degradation of low density lipoprotein (LDL) via the LDL receptor as well as oxidized species of cholesterol. Competitive inhibitors of the reductase induce the expression of LDL receptors in the liver, which in turn increases the catabolism of plasma LDL and lowers the plasma concentration of cholesterol, an important determinant of atherosclerosis. Where is HMG-CoA reductase in the cell? HMG0-CoA reductase is anchored in the membrane of the endoplasmic reticulum, and is long regarded as having seven transmembrane domains, with the active site located in a long carboxyl terminal domain in the cytosol. What is the precursor of squalene? Mevalonate becomes squalene, but must first be converted into two 5-C intermediates called isopentenyl pyrophosphate and dimethylallyl pyrophosphate, which join to yield farnesyl pyrophosphate. Two farnesyl pyrophosphate combines into squalene. What is the precursor of steroid hormones? Give examples for steroid hormones. Cholesterol is the precursor to all bile acids and all steroid hormones. Examples of steroid hormones include sex hormones such as estradiol, testosterone and other androgens and estrogens, and progestagens, corticosteroids including glucocorticoids and mineralocorticoids, anabolic steroids, and cholesterol itself. What is the precursor to isoprenoid compounds? Isoprenoids are derived from five-carbons isoprene units assembled and modified in thousands of ways. Many isoprenoids in the nature, including cholesterol in animals, are synthesized using mevalonate- based isopentenyl pyrophosphate biosynthetic pathway. Formation of mevalonate is key for isoprenoid biosynthesis. Which enzyme removes most of the cholesterol side chain? Cholesterol can be fatty acylated to form cholesteryl esters in all cells, or it can be oxidized to form oxysterols by enzymatic reactions or by auto-oxidation in all cells or oxidized to bile acids in hepatocytes only (this is relevant for the net elimination of sterols from the body) or oxidized to steroid hormones in steroidogenic cells. Key enzymes are ACAT, acyl CoA cholesterol acyltranferase; CYP7A, cholesterol-7 - hydroxylase; P450SCC, cholesterol side-chain cleavage enzyme involved in steroidogenesis. I looked online and found that it was desmolase, the first reaction of steroid synthesis. It seems to make sense, see slide 147. Desmolase in the mitochondria does seem to remove most of the cholesterol side chain. What is/are the product of cholesterol degradation? Bile acids and steroid hormones What is the precursor of bile acids? Cholesterol is the precursor of bile acids. 1). Oxidation of cholesterol by a mixed-function oxidase in which cholic acid is formed by the action of 7a-hydroxy-cholesterol. 2). Conjugation with taurine or glycine produces taurocholic acid and glycocholic acid, respectively. What is the function of bile acids? Facilitate the formation of micelles, which promotes processing of dietary fat. Essential for the digestion of food, especially for solubilization of ingested fats, cholesterol, and fat-soluble vitamins. Which organ secretes bile acids? Bile acids are secreted from liver, but stored in the gall bladder. What are the two common bile acids? Glycocholic acid (from glycine) and taurocholic acid (from taurine) acid How are triglycerides, phospholipids and cholesterol distributed in the body? Triglycerides are a mechanism for storing unused calories, and their high concentration in blood correlates with the consumption of starchy and other high carbohydrate foods. They are found in adipose tissue. Fat and liver cells can store and synthesize triglycerides. Phospholipids are a class of lipids that are a major component of all cell membranes as they can form lipid bilayers. Phospholipid synthesis occurs in the cytosol adjacent to ER membrane Cholesterol is an important component in mammalian cell membranes. It is formed in the liver and can be found in the bloodstream. Cholesterol is recycled. It is excreted by the liver via the bile into the digestive tract, in a non-esterified form. Typically about 50% of the excreted cholesterol is reabsorbed by the small bowel back into the bloodstream. When saying “distributed”, I thought they meant transported, in which the answer would be lipoproteins. This is another answer as well. I thought that this question asked where in the body can you find cholesterol, phospholipid, triglycerides. What is the function of chylomicrons? Chylomicrons form in the intestines. Their main task is to carry triglycerides. Dietary lipids are packaged in the intestine into chylomicrons for transport in the blood stream. What are the two sources of cholesterol in mammalians? The first source is diet: Animal fats are complex mixtures of triglycerides, with lesser amounts of phospholipids and cholesterol. As a consequence, all foods containing animal fat contain cholesterol to varying extents. Major dietary sources of cholesterol include cheese, egg yolks, beef, pork, poultry, fish, and shrimp. Human breast milk also contains significant quantities of cholesterol. The second source is biosythesis: All animal cells manufacture cholesterol with relative production rates varying by cell type and organ function. About 20–25% of total daily cholesterol production occurs in the liver; other sites of higher synthesis rates include the intestines, adrenal glands, and reproductive organs. Know the "general" structure and/or structural backbone of: A compound ending in -ate just means that the hydrogens are stripped off from -COOH groups (Phospho)pantethenic acid (has an amide bond, and has primary and secondary hydroxyl groups) Citrate (three carboxylic groups, and a tertiary alcohol) Oxoacetate (4-carbon dicarboxylic acid) Pyruvate (three carbon, alpha-keto acid) Succinate (a 4-carbon dicarboxylic acid, but no keto group like oxaloacetate Remeber that succinate doesn't have an O, so it has no keto group. Acetyl-CoA Acetate (two carbon carboxylic acid) Mevalonate (take away the three H's from the three -OH's) 3-hydroxyl-3methylglutaryl (HMG) / HMG - CoA CoA HMG CoA Isopentenyl pyrophosphate Pyrophosphate Cholesterol (3 six-carbon rings, and one five-carbon ring, and a long alkyl chain attached) Steroid hormones (the general structure) Prostaglandins (look for one or two five-membered rings, and two long alkyl chains) Prostaglandin I2, prostacyclin Prostaglandin E2, alprostadil Chapter 27 Integration of Metabolism and Organ Specialization What are the evolved principles of metabolism? 1. Maximize the efficiency of fuel utilization by preventing the simultaneous operation of opposing pathways (i.e., futile cycle) 2. Partition metabolites appropriately between alternative pathways. 3. Draw on the fuel best suited for the immediate needs of the organism. 4. Shut down biosynthetic pathways when their products accumulate. What are the key intermediate compounds that interconnect major metabolic blocks? Carbolic and anabolic pathways, occurring simultaneously, must act as a regulated, orderly, responsive whole. Just a few intermediates connect major systems - sugar-phosphates (3-C to 6-C), alpha-keto acids like oxaloacetate and pyruvate, alpha-ketoglutarate, acetyl-CoA and Succinyl-CoA, and phosphoenolpyruvate (PEP). Slide 89 will help explain each of the compounds and where they come from. Which compounds couple the energy production in catabolism with the energy using processes in anabolism? ATP & NADPH couple catabolism and anabolism becuase they are recycled and not replaced. What is the evolved ATP coupling stoichiometry? There are three types of stoichoimetry of ATP: simple reaction, obligate coupling, and evolved coupling Evolved coupling stoichoimetry is the number of ATP molecules that pathways have evolved to consume or produce. For example, the value of 38 ATP cannot be predicted from any chemical consideration and is an end result of biological adaptation. It is a trait that evolved through interactions between chemistry, heredity, and the environment over the course of evolution. The number is high enough for energy, but low enough for all the glucose to be used. What is the ATP coupling coefficient? The coupling coefficient is the moles of ATP produced or consumed per mole of substrate converted or (or product formed) Cellular oxidation of glucose has a coupling coefficient of 30-38 (depending on cell type) Hexokinase has coupling coeffcient of -1 (one ATP used). Pyruvate kinase (in glycolysis) has a coupling coefficient of +1. How is ATP hydrolysis (ATP --> ADP + Pi) and (ATP --> AMP + PPi) coupled to metabolic reactions requiring energy input? ATP hydrolysis is accompanied by a relatively large decrease in free energy. This decrease in free energy can be used to drive other energy-requiring reactions within the cell. Actual intracellular concentrations of inorganic phosphate are approximately 10 M, and so intracellular concentrations of ATP are higher than those of ADP. This favors ATP hydrolysis within a cell. An example is shown on slide 18 dealing with acyl-CoA synthetase reaction. PPi can be broken down to give off energy, and may provide the needed energy that ATP hydrolysis alone cannot provide. Living cells avoid big changes in free energy because a strong thump from a violent reaction may be not endured by enzymes. Living cells use ATP to nudge the reaction forward in the desired direction. What is the most critical matter in terms of ATP supply for a eukaryotic cell during high demand for energy? ATP supply needs to be kept high. The ratio of ATP:ADP in the cytosol is typically 200:1 or more, and it is the lifetime of ADP rather than ATP which is critical for success. In energy-demanding conditions, what matters is the maximum possible reaction flux. Diffusion rate is proportional to concentration, so the real bottleneck is returning the "empties" for recycling. The minuscule amount of ADP in working muscle has only milliseconds to get back from the contractile proteins to the mitochondria for reconversion into ATP. What is the function of adenylate kinase? Catalyzes this reaction: ATP + AMP --><-- 2 ADP How is AMP-activated protein kinase (AMPK) activated? If the cell is subjected to a metabolic stress that interferes with ATP synthesis or a stress that accelerates ATP consumption, the ADP/ATP ratio tends to increase. This is amplified by adenylate kinase into a much larger increase in the AMP/ATP ratio that then switches on AMPK. AMPK restores energy homeostasis by promoting catabolism and inhibiting ATP consuming process. Basically, low energy status (high AMP or low ATP) activates AMPK., which phosphorylates many targets controlling cellular energy production and consumption. The kinase is activated in response to stresses that deplete cellular ATP supplies such as low glucose, hypoxia, ischemia and heat shock. How are the pools for ATP, ADP and AMP maintained in the cell? Adenylate kinase reaction provides a direct connection among all three members of the adenylate pool. What are the two roles of ATP? 1. ATP is the energy currency of the cell, establishing large equilibrium constants for metabolic conversion and render metabolic sequences thermodynamically favorable. 2. Energy transduction and energy storage. Described as the”adenylate system”, which refers to the adenine nucleotide pool in the form ATP, ADP and AMP. Uncontrolled changes in adenylate system can have drastic consequences. It also serves as allosteric regulation and stoichiometric involvement. What is energy charge (E.C.)? Energy charge provides a measure of how fully charged the adenylate system in terms of high-energy phosphoryl groups. It has a range of 0 to 1.0, and is a ratio of adenylate phosphoric anhydride bonds to total adenylate concentration. When all of the adenylate is ATP, the EC is 1. The energy charge is the fraction of usable high energy bonds in the total adenine nucleotide pool. It is an index of how fully charged adenylates are with phosphoric anhydrides. How do regulatory enzymes respond to energy charge? •Regulatory enzymes respond in predictable ways to energy charge. •Enzymes in catabolic, energy producing, ATP-generating pathways (R enzyme activities) are more active at low E.C. •Enzymes in anabolic energy consuming reactions, ATP-utilizing (U enzyme activities) are more active at high E.C. What is the energy charge at steady-state metabolism? The reciprocal relationship between R and U systems means that energy charge reaches a point of metabolic steady-state at an E.C. value of 0.85 - 0.88. The E.C. in healthy cells normally oscillates between 0.85 and 0.88. What is the phosphorylation potential? The phosphorylation potential, , where ΔGO′ is the standard free energy of hydrolysis of ATP at a given pH, and [ATP], [ADP] and [Pi] refer to concentrations in the suspending medium, has been determined in rat-liver mitochondria under various conditions. Phosphorylation is equal to( [ATP]/([ADP][Pi])). It varies significantly, unlike energy charge, ranging from 200 to 800 M-1, or more, with higher levels signifying more ATP and correspondingly greater phosphorylation potential. What are the metabolic relationships among human organs? Glycogen is stored in the liver and muscle, triacylglycerols (fats) are stored in adipose tissue, and protein is mostly in skeletal muscle. Carbs > Fats > Proteins is the order of energy use for the body. Brain Very demanding for oxygen but independent for mental activity (brain does nothing while it sleeps but still needs oxygen); has no fuel reserves at all. It uses only glucose in the blood and depends on the blood flow completely. The glucose is used for ATP synthesis and is needed to power plasma membrane sodium-potassium ATP-ase to maintain membrane potential and nerve impulse transmission. Fat can be converted to beta-hydroxybutyrate in the liver and used by the brain and glucose from gluconeogenesis in the liver can be used as well, though this won't occur until all the fat has been used up. Muscle Uses 30% of oxygen at rest but can account for 90% of total metabolism during maximal exertion. Muscle metabolism is a demand response. Muscles can use fatty acids, glucose, and ketone bodies for energy. Muscle relies on phosphocreatine for 4 seconds and then uses glycogen to get its ATP. After 20 seconds, pH levels and PFK drop in the muscle and so this causes lowered flux of hexose causing fatigue. Amino acids can be generated from muscle if it gets to that point. Heart Constant and rhythmic action, so the range of activity is less than muscle. The heart is a completely aerobic organ and has lots of mitochondria. The heart prefers fatty acids as fuels and doesn't have much reserves, only some phosphocreatine and some glycogen. Thus, it needs the oxygen and free fatty acids, glucose, or ketone bodies as fuel. Adipose tissue Primarily long-term energy storage, but they also have metabolic activity. They need the glucose to make NADPH and make triglycerides, using fatty acids from the liver. If glucose levels are down, then free fatty acids are released. If glucose levels are up, then they are converted into acetyl-CoA for fatty acid synthesis. Brown fat Brown fat has lots of mitochondria, thermogenin to make the proton channel and gradient heat producing instead of ATP producing. Brown fat can be found in newborns and hibernating animals. Liver Converts G-6-P to glycogen, released as blood glucose, used to generate NADPH and pentoses via the pentose phosphate cycle, or catabolized to acetyl-CoA for fatty acid synthesis or oxidative phosphorylation. It buffers the level of blood glucose by storing it as glycogen and breaking it down. Fatty acid turnover occurs here, as triglycerides are broken down and fatty acids are degraded in the liver to acetyl-CoA to form ketone bodies. The liver can also make cholesterol from acetyl-CoA and deposit triglycerides as fat. The liver can use amino acids as fuel via amino transferases, and turning them into alpha-keto acids to be used in the cellular respiration. How are the signals for food intake and energy expenditure coordinated in the brain? There are two sets of neurons in the arcuate nucleus that regulated by circulating hormones. Agrp and Npy are neuropeptides that stimulate food intake and decrease energy expenditure. Melanocyte stimulating hormones and Cart are neuropeptides that inhibit food intake and increase energy expenditure. What are the neuroendocrine hormones involved in the regulation/integration of energy metabolism in the brain? ● GABA (preoptic area) inhibits Pomc/Cart neurons ● Ghrelin (stomach) stimulates Npy/Agrp neurons ● Leptin (adipose tissue) and Insulin (pancreas) inhibit Agrp/Npy neurons and stimulate adjacent Pomc/Cart neurons ● YY3 – 36 inhibts NPY/Argp What is the function of the orexin system? How is it regulated by different stimuli? Orexin system is in the lateral hypothalamus. They are excitatory neuropeptides that promote wakefulness and stimulate food intake through activating all the components of the ascending arousal system including cell groups in the brain stem and hypothalamus. Excitatory effects result in increased in sympathetic nervous system. The orexin system also activates the appetite-promoting neuropeptide Y neurons in the hypothalamus leading to increased hedonic homeostatic feeding. The orexigenic and anorexigenic signals that are produced by the NPY/AGRP and POMC/CART neurons are then sent to second-order downstream effector neurons, which also receive modifying inputs from dopamine, serotonin and endocannabinoid signals. These effector neurons express receptors that include the Y1 receptor (Y1R) and the melanocortin 4 receptor (MC4R). These various inputs come together to provide the overall balance between food intake and energy expenditure. What are the hormones secreted by peripheral tissue/organs that influence the orexin system? The orexin system is regulated by ghrelin (stimulated) from stomach, and inhibited by leptin (adipose tissue), sleep, preoptic area, and GABA. Insulin from the pancreas and PYY3-36 also influence the orexin system. What is the function of creatine kinase? It is difficult to maintain a very high ATP/ADP ratio, so creatine and creatine phosphate are both highly diffusible energy carriers to solve this problem. Creatine kinase and phosphocreatine act as a buffer system, providing additional ATP. Creatine phosphokinase in the mitochondria phosphorylates creatine into creatine phosphate. However, when lots of ADP is around from ATP hydrolysis, creatine kinase in the muscles catalyzes the formation of ATP at the using phosphocreatine as the phosphate source. During rest, ATP levels are high again, so creatine kinase acts in reverse to restore the phosphocreatine supply. How does muscle protein degradation feed the energy demand in the muscle? How is the amino acid alanine converted into a metabolic fuel pyruvate? Amino acids are degraded to pyruvate, which can be transaminated to alanine. Alanine circulates to liver, where it is converted back to pyruvate, which feeds into gluconeognesis. This is a fuel of last resort for starving organisms. Important note: I believe that the reason this provides energy is because the conversion of pyruvate to alanine accompanies the conversion of glutamate into a-ketoglutarate which can be used for energy. Looking at the summaries of Chapter 24 and 27 may help in know what may be asked in the exam. [Show More]
Last updated: 1 year ago
Preview 1 out of 20 pages
.png)
Also available in bundle (1)

BIOL 3362 - Exam 1 -4 Study Guide BUNDLE | Download To Score A.
BIOL 3362 - Exam 1 -4 Study Guide BUNDLE | Download To Score A.
By A+ Solutions 2 years ago
$14
5
Reviews( 0 )
Document information
Connected school, study & course
About the document
Uploaded On
Sep 22, 2021
Number of pages
20
Written in
Additional information
This document has been written for:
Uploaded
Sep 22, 2021
Downloads
0
Views
46











.png)











.png)






