BioChemistry > STUDY GUIDE > BIOL 3362 - EXAM 1 STUDY GUIDE (Biochemistry, Photosynthesis, Amino Acids). (All)
BIOL 3362 - EXAM 1 STUDY GUIDE (Biochemistry, Photosynthesis, Amino Acids).
Document Content and Description Below
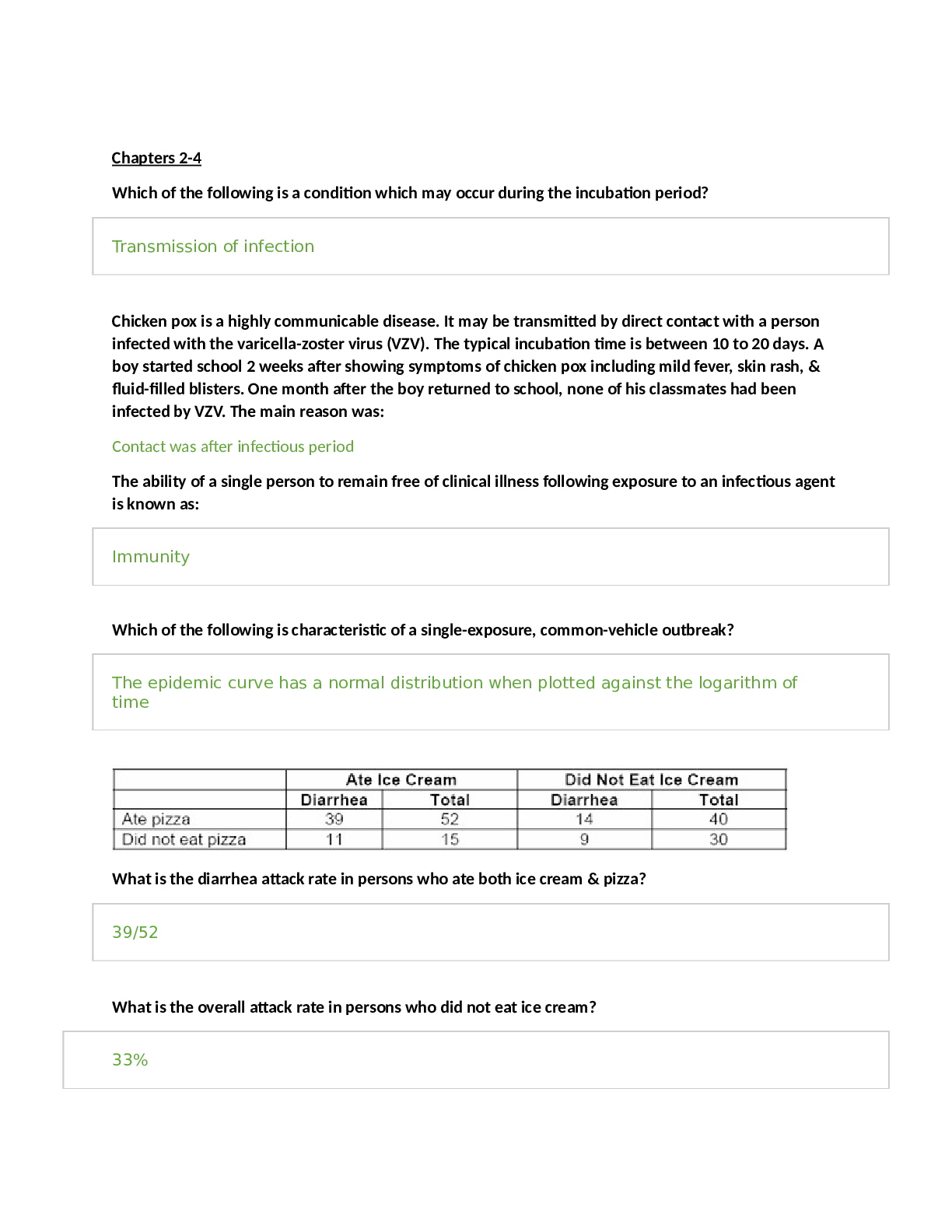
STUDY GUIDE TEST 1 CHAPTER 21: PHOTOSYNTHESIS 1. Where does photosynthesis occurs in plants and bacteria? • Photosynthesis occurs in membranes • Plants: Membranes are in large organelles c... alled chloroplasts • Bacteria: photosynthetic bacteria, the membranes are major components of the cytoplasm 2. Chloroplasts structure and function are very important. • Chloroplast: plastids, a group of related organelles • Inner membrane system called thylakoid membranes (flattened discs or sacs) • 3 inner membrane space aqueous regions (stroma, innermembrane space, and thylakoid lumens) • Chloroplasts contain DNA, RNA, and Ribosomes 3. What are light and dark reactions? • Light Reactions: Illuminated chloroplasts without CO2 will generate oxygen, NADPH, and ATP once illuminated, they can be placed in the dark and fix CO2= Dark Reaction • Dark Reactions occur in the stroma • Light Reactions occur in the thylakoid membranes 4. What are the products of photosynthesis and how are they made? What is the source of electrons? • Hexose+ C6H12O6 +6O2 are products of photosynthesis, and they are made by photosynthesis evolving O2 and generating energy used to fix CO2 • The NADPH and ATP are used for CO2 fixation, and can drive the endergonic process of hexose formation • Water is the electron source for NADPH and ATP synthesis. See equation 21.2 on p 633. In bacteria, the electron source may also be H2S (photosynthetic green and purple sulfur bacteria), isopropanol, or other oxidizable (reduced) compounds. • Light must provide the energy for NADPH synthesis, which must exceed 219 kJ/mol. 5. What is chlorophyll? What is its function? What does it contain (in terms of metals, and functional groups)? • Chlorophyll- Magnesium-containing substituted tetrapyrroles (Fig 21.5a). Similar to heme, but differing with respect to the metal ion, a long-chain alcohol (phytol), and a fifth five-membered ring. • They are good light absorbers because of delocalized π electrons above and below planar rings. Visible light absorption promotes an electron to a higher orbital, which can be transferred to another compound. This is a reduction reaction. • The absorption of chlorophylls a and b differ (Fig 21.5b), which along with accessory light-harvesting reactions (Fig 21.6) permit absorption of a variety of wavelengths. Note the conjugated double bond system. Carotenoids also destroy reactive oxygen species. Leaf colors during autumn are due to persistence of some of these pigments. 6. What are fates of excited electrons? • The excited electron has four fates 1) Heat loss (e– goes to lower state and increases vibrational energy) 2) (Loss as Light) Fluorescent light (at a longer, less energetic wavelength). This occurs only for saturating light. 3) Resonance energy transfer (excitation of e– of neighboring molecule). This process permits energy transfer to one reactive site (described below). An exciton is said to be transferred. 4) Energy transduction. The more energetic molecule is a better electron donor (reducing agent). This is the transfer of light energy to chemical energy. 7. What is a reactive center? • Reaction Center- special pair of photochemically reactive chlorophyll a molecules • For algae, only one O2 made for every 2400 chlorophyll molecules (quantum yield). There is only one reaction center which contains only two chlorophyll a molecules. Most chlorophyll molecules transduce energy to the reactive center (Fig 21.9). • The logic of energy transfer is as follows (Fig 21.8). o Light causes loss of electron from chlorophyll. o Energized electron is used for synthesis of NADPH (a reducing agent) o Cationic chlorophyll (minus electron) extracts electron from H2O, which produces O2. 8. What are the two photosystems and what are their functions? What is the Z scheme? • Photosystem II (PSII) has maximal absorption at 680 nm. It evolves O2. It contains the reaction centers also. It uses quinones as terminal electron acceptors in the complex. • Eukaryotes have both, while photosynthetic bacteria (excluding cyanobacteria) have PSII but do not generate O2. • P700 and P680 are the reaction centers of PSI and PSII, respectively. Both are chlorophyll a dimers and are present in small amounts (~0.25%) of total chlorophyll. A small amount of pigment is bleached (losses color) when illuminated. Same result is seen with addition of an e– acceptor. Therefore, bleaching correlates with e– loss. • There are three types of chlorophylls: those in the light-harvesting complex (chlorophyll a or b), those in PSI, and those in PSII. These complexes can be physically separated. • The photosystems have different functions (Fig 21.10): • PSI is a very strong reducing agent (e– donor) that reduces NADP to NADPH, which itself is a strong reducing agent. • PSII is a very strong oxidizing agent (e– acceptor) that splits H2O, which produces O2. • PSII transfers e– to PSI. This process pumps protons via the electron transport chain, which can be used for ATP synthesis. • Notice the separation of reducing and oxidizing agent. Electrons flow from H2O to NADP+, driven by light. Oxygen is simply a by-product. • Photosynthetic electron transfer: the Z scheme (Fig 21.11 top) 9. What are the similarities between electron transport in oxidative phosphorylation and photosynthesis? What components are in common? • Electron transfer follows a Z scheme (up-down-up) with respect to reduction potentials. The up changes require energy input. • The sequence involves e– transfer from water via the water splitting component, to activated P680, to quinones (plastoquinones), to an Fe-S center in cytochrome f, to plastocyanin, to P700, to membrane-bound ferrodoxins, to soluble ferrodoxins, to a reductase, to NADPH. Note the similarity to e– transport in oxidative phosphorylation. 10. Ferrodoxin shows up a lot. Why? • Soluble Ferrodoxin is an electron donor to flavoprotein (Fp) that catalyzes NADP+ Reduction (Ferrodoxin: NADP+ Reductase), which is needed in the process of photosynthesis. 11. What is the difference between cyclic and noncylic photophosphorylation? • Cyclic photophosphorylation- shows a way to recycle e– that can be used for ATP synthesis. It has an additional protein, cytochrome b6. • Noncyclic photophosphorylation is shown in Fig 21.20. This shows the Z scheme of e– transfer. • Cyclic photophosphorylation differs in that the e– comes from the P700 of PSI (Fig 21.22). The e– is never donated to NADP+. The e– involves plastoquinone, so that a proton is pumped into the lumen. O2 is not evolved. This process is 20 times slower than noncyclic photophosphorylation. This may overcome an ATP deficit produced by the Z scheme. 12. Where are protons pumped in the process? What steps, and into what compartments? • O2 evolution and PSII states. O2 is evolved after four flashes of light. Each step generates an e– and a proton. P680 exists in five oxidation states, called S0 through S4. O2 is released when S4 is reached. • P680* donates e- to pheophytin, chl a (except 2 H+ replaces Mg++), to specialized plastoquinones, to soluble plastoquinones (a shuttle from PSII to cyt b6f). PQ is an analog of coenzyme Q. • The e– transfer involves proton transport into thylakoid lumen, which creates PMF. This occurs during recycling of plastoquinone, and by cyt f. • The electron transfer is very rapid to prevent reassociation of positively charged molecules and the electrons. • Oxygen evolution: light removes four e- from a metal cluster (with four Mn); 2 H2O replace the lost e-s. 13. How were chloroplasts used to demonstrate the chemiosmotic mechanism of ATP synthesis? • The first demonstration of the chemiosmotic mechanism of ATP synthesis was with chloroplasts. An artificial pH gradient was shown to be sufficient for ATP synthesis (diagram in class is from the third edition, but omitted in later editions. 14. Carbon dioxide fixation. Which reactions incorporate CO2? What are the unique reactions of the Calvin-Benson cycle? Where are ATP and NADPH used? • The point of photosynthesis is to synthesize compounds, and hexoses are synthesized from CO2. Although there are reactions that consume CO2 (aneuplerotic reactions of the TCA cycle), they do not accumulate organic material. One of the first uses of radioactive isotopes was the demonstration that 14CO2 went into 3-phosphoglycerate. • The CO2 acceptor is ribulose-1,5-bisphophate, a five-carbon intermediate. A six- carbon intermediate is generated, which is cleaved to two molecules of 3- phosphoglycerate • Calvin –Benson Cycle-: transforms 3-phosphoglycerate (3PG) to hexoses. The reactions consume the ATP and NADPH generated by the light reactions (Fig 21.25). The net synthesis of one hexose from 6 CO2 requires 18 ATP and 12 NADPH. The cycle performs three important functions: a) CO2 fixation b) Reduction of 3PG to glyceraldehyde-3-P (GAP) for carbohydrate synthesis c) Conversion of 3-carbon units to 4-, 5-, 6-, and 7-carbon units. The Calvin Benson Cycle also: • Many of the reactions are catalyzed by glycolytic and pentose cycle enzymes. The unique reactions are: • Rubisco • P-glycerate kinase (actually a glycolytic enzyme). It consumes 12 ATPs. • NADPH-dependent GAP dehydrogenase (note that a glycolytic enzyme uses NAD and catalyzes the reverse reaction). This accounts for all the NADPH consumed by the Calvin cycle. • The carbon rearrangements are mediated by transketolase and aldolase, which works in reverse of glycolysis and can generate a 7-carbon intermediate. A phosphatase acting on S-1,7-bisP is unique to plants. • Finally, ribulose-5-P kinase to make RuBP, which accounts for 6 ATPs. 15. What are the reactions of Rubisco? How is Rubisco activity controlled? • The CO2 acceptor is ribulose-1,5-bisphophate, a five-carbon intermediate. A six-carbon intermediate is generated, which is cleaved to two molecules of 3-phosphoglycerate. • The enzyme catalyzing CO2 fixation is ribulose bisphosphate carboxylase/oxygenase (rubisco for short). It reacts with either CO2 or O2. • It is located in chloroplast stroma, and may be the most abundant protein in nature. • It has an α8β8 structure. • The large subunit is catalytic, the small subunit regulatory. • Regulation is complex. • The enzyme is carboxylated on a specific lysine, which generates a carbamyl group (–NH–COO–). This permits binding of Mg++, which activates the enzyme. • RuBP inhibits (binds tightly to the non-carbamylated form). A light-activated activase removes RuBP. Therefore, light activates rubisco. • Km for rubisco for CO2 is about 10 μM, which is also the concentration of solute CO2 in equilibrium with air (which is 0.03% CO2). • 16. How does light control CO2 fixation? Thioredoxin and ferrodoxin involvement is this control. • Light induces CO2 fixation indirectly. It causes increased pH in the stroma, which activates rubisco and ribosco activase, and other key enzymes (Fig 21.27). • Light increases reducing power. Thioredoxin carries e–s between reduced ferrodoxin and NADPH. It also reduces certain disulfide bonds in key enzymes (FBPase, SBPase, and Ri-5-P kinase) which activates them. Thioredoxin is a small protein. • Light increases Mg++ efflux from the lumen to stroma, which also activates several enzymes, including FBPase, which may be the rate limiting step in CO2 fixation. 17. How do plants avoid the problem of the reaction of Rubisco with oxygen? • An unstructured protein, CP12, interacts with and inhibits Rib-5-P kinase and GAP DH. This blocks ATP and NADPH utilization. Reduced thioredoxin prevents the interaction. • RuBP oxygenase activity (Fig 21.29). If O2 is used instead of CO2 by rubisco, then the products are 3PG and glycolate. • This reaction occurs at about 25% of rate of CO2 fixation • Glycolate can be converted to glyoxylate, and then to glycine and serine. The conversion to serine releases CO2. This is called photorespiration since CO2 is released. • The C-4 pathway of CO2 fixation (Fig 21.30) • Tropical grasses incorporate 14CO2 into malate or aspartate instead of 3PG. Malate and aspartate act as a CO2 delivery system to take CO2 from the oxygen rich surface to the oxygen-poor interior. This avoids the problem with O2 reaction with rubisco. • Mesophyll cells (at surface) incorporate CO2 into OAA, which is catalyzed by PEP carboxylase. OAA is converted to malate by an NADPH-dependent MDH or to aspartate by transamination. • Malate or aspartate is transferred to bundle sheath cells (interior). Malate is then decarboxylated to pyruvate. The CO2 liberated is used in the Calvin cycle. • The pyruvate is then transferred back and PEP formed (using ATP). The enzyme is not a reversal of pyruvate kinase, but is pyruvate-Pi dikinase. Both Pi and pyruvate are phosphorylated. • This pathway has separated CO2 uptake and fixation in different compartments. It is much more efficient. CHAPTER 25: Nitrogen acquisition 1. The nitrogen cycle: what are nitrate assimilation, nitrogen fixation, nitrification, denitrification? • Nitrate assimilation is the conversion of nitrate to ammonia. It occurs in plants, some fungi, and some bacteria. • Nitrogen fixation is the conversion of N2 to ammonia. It occurs only in bacteria. Sometimes these bacteria associate with plants. This is an anaerobic process. • Animals can do neither, so they must acquire nitrogenous compounds from either plants or bacteria. • Animals release nitrogen in a reduced form: ammonia, urea, or possibly uric acid. This release occurs to dispose some waste products or as decomposition. • Nitrification. Bacteria then can take the reduced nitrogen, as ammonia, and oxidize it. Nitrifying bacteria convert ammonia to nitrite and nitrate, and use the energy released for growth. This process is called nitrification because it makes the nitrogen more readily available to plants. Nitrate is more soluble than ammonia, and plants absorb it more quickly. • Denitrification is the conversion of nitrate to N2, which is released to the atmosphere. It is done by denitrifying bacteria, which are very efficient. The function of this process is to provide bacteria with an electron acceptor other than oxygen for energy generation. This is an anaerobic process. Oxygen is a preferred electron acceptor, and shuts this off in E. coli. Furthermore, oxygen interfers with this process. 2. The steps of nitrate assimilation: how many electrons involved and what metals are involved? a. What are the source of electrons? • Nitrate assimilation occurs in two steps (see equations on p 842). • The first step is a two-electron reduction of nitrate to nitrite, catalyzed by nitrate reductase. • The second step is a six-electron reduction of nitrite to ammonia, catalyzed by nitrite reductase. • Nitrate reductase transfers a pair of electrons via four protein-bound intermediates to nitrate (see diagram on p 842). • NADH to SH to FAD to cyto b557 to MoCo and finally to nitrate. • MoCo is a molybdenum-containing cofactor (see Fig 25.2a) MoCo is a cofactor for a variety of hydroxylase-type reactions: xanthine DH, aldehyde oxidase, sulfite oxidase. • Nitrite reductase (plants) obtains electrons from photosynthetically reduced ferrodoxin. Electrons transferred to a 4Fe-4S cluster to siroheme (Fig 25.2b) and finally to nitrite, which binds the siroheme and accepts electrons from it. Microbial nitrite reductases have more complex electron transfer pathways. 3. Nitrogen fixation and nitrogenase. What organisms do this? What are its requirements (reductant, energy, etc)? What are its components (the two protein components), and what do they do? a. What factors control and affect its activity? • Nitrogen fixation (see equation p 843) reduces N2. The enzyme nitrogenase is only found in bacterial cells. Eight electrons are transferred, and one molecule of H2 is obligatorily produced. • It is the only mechanism that provides direct access to the vast reserves of nitrogen gas in the atmosphere. • N2-fixing bacteria can associate with higher plants. This is important since nitrogen availability often limits plant growth. • All nitrogenases require the enzyme nitrogenase, a strong reductant (such as reduced ferrodoxin), ATP, and O2-free conditions. • The enzyme contains two components: the Fe-protein (nitrogenase reductase) and the MoFe-protein (nitrogenase) • The reductase hydrolyzes two ATP per electron transferred, or 16 per N2. The ATP is used for overcoming the activation energy required to break the N2 bonds (Fig. 25.4). This component has a single 4Fe-4S cluster, and is very O2 sensitive. • The nitrogenase component is an 22 protein. Each dimer contains a P-cluster (8Fe-7S cluster – see Fig 25.5a) and the FeMo-cofactor (a 7Fe-1Mo-9S cluster – see Fig 25.5b). This component is also O2 sensitive. • The reaction involves a complex electron transfer (Fig 25.6). It starts with reduced ferrodoxin, which has a greater redox potential than NADPH. The reductase is the electron donor. ATP hydrolysis is coupled to electron transfer from the reductase to the nitrogenase component. The process is slow, about 3 N2 per second, so that cells may contain large amounts of the protein, about 5% of total. • Two factors commonly control nitrogenase (Fig 25.8): • ADP inhibits activity. • NH + inactivates a transcriptional activator. • Some organisms have a third mechanism, ADP-ribosylation of the nitrogenase reductase subunit. 4. What pathways assimilate ammonia and what are the products? What are the differences between the two pathways. What is the reductant? • The GDH pathway. This pathway synthesizes glutamate and glutamine by the actions of GDH and GS (Fig 25.11). GDH has a high Km for ammonia (~2 mM in E. coli), whereas GS has a much lower Km (~0.1 mM). With high ammonia (which is not normal in nature), GDH can assimilate ammonia into glutamate, and GS can assimilate ammonia into glutamine. • The GS-glutamate synthase pathway (Fig 25.13). When ammonia is low, which is normal in nature, this pathway first synthesizes glutamine via GS from ammonia and glutamate. Then, glutamate synthase, uses glutamine as a nitrogen donor for the formation of glutamate (Fig 25.12). The difference in this pathway is energy consumption. The GDH pathway does not need to consume ATP for glutamate synthesis. The GS-glutamate synthase pathway does. It can be calculated for E. coli that the difference in energy is 10% of the cell’s total energy requirement. • The primary differences are that one requires more energy than the other, and the former can assimilate a low concentration of ammonia. • NADPH is the reductant 5. Know the reaction mechanism of glutamine synthetase since is is found for many enzymes. 6. What are the intracellular nitrogen donors, and their relative quantitative importance. • In considering these pathways, they are not used as described in the book. With high ammonia, the cell needs 75% of its nitrogen as glutamate, and 25% as glutamine. Therefore, the GDH pathway assimilates 75% of the ammonia, and GS 25%. With low ammonia, the GS-glutamate synthase pathway and GS assimilates 100% of the ammonia, and converts 75% of the glutamine made to glutamate. 7. How is glutamine synthetase controlled? a. Principles of feedback inhibition, the covalent modification, and gene expression controls: what metabolites are involved? (I will not ask specific inhibitors, but what properties are shared by the inhibitors.) b. What is the effect of high and low glutamine on these controls? • Glutamine synthetase is controlled by feedback inhibition, covalent modification, and gene expression(which determines how much is made) Feedback Inhibition: Gly and Ser will accumulate if the cell has enough purines, which require glutamine for their synthesis. They are therefore indirect indicators of glutamine status. Why alanine inhibits is not known. These amino acids bind to the glutamate site. The other compounds require glutamine for their synthesis, and are endproducts. Two of them also bind to substrate sites: AMP and carbamoyl~P. The others bind to allosteric sites. Covalent Modification: inactivates GS. ATase is an enzyme that adds and removes adenylyl groups depending on the environment. The mechanism of this control is stated incorrectly in the text. Glutamine allosterically controls a bifunctional regulatory enzyme, uridylyltransferase (UTase)/uridylyl-removing enzyme (UR) which controls the activity of another regulatory protein, PII, which in turn controls ATase activity. With high glutamine, UR is active, which results in PII formation (instead of PII-UMP). PII interacts with ATase and adenylylates GS. This form of PII is called PIIA. With low glutamine, UTase is active, and PII-UMP is formed. Its interaction with ATase results in deadenylylation of GS. This form of PII is called PIID. -ketoglutarate does have an effect, but only with unmodified PII. Therefore, the story is complicated. Glutamine is the major effector. It is not as the book describes. Gene Expression: High glutamine (nitrogen excess), which results in PIIA, results in interference of phosphate transfer from NRII to NRI. NRI~P is a transcriptional activator, and the unphosphorylated form is inactive. The gene for GS is not expressed. Low glutamine (nitrogen limitation) has the opposite effect. It results in PIID formation, and net transfer of phosphate from NRII which phosphorylates itself and transfers phosphate to NRI. NRI~P is a transcriptional activator that turns on the gene for GS. Despite what the book says, it is the level of glutamine that is important, not the ratio of glutamine to -ketoglutarate. 8. Transaminations: nitrogen donor, cofactor, reversibility Transaminations: Amino acid synthesis frequently involves addition of a nitrogen from glutamate to an -keto acid by transamination (Fig 25.19). The process involves transfer of an -amino group from a donor, usually glutamate, to the -keto position of an -keto acid. Amino acid 1 (usually glutamate) + -keto acid 2 -keto acid 1 (usually - ketoglutarate) + amino acid The enzymes are named for their amino acid substrates, e.g., glutamate- aspartate aminotransferases. The reactions are usually readily reversible. Glutamate is virtually always in excess, which determines the direction of the reaction. The aminotransferases require pyridoxal phosphate as an essential cofactor. The intermediate pyridoxamine donates the amino group (p 856). 9. Amino acid classifications: families, and members of each family • The -ketoglutarate family • This family includes glutamate, glutamine, proline, and arginine. In fungi, this family also includes lysine. • We already discussed glutamate synthesis as part of the mechanism of ammonia assimilation. • The central fact about members of this family is that the modifications occur on the - carboxyl group of glutamate. The major changes are often at this carbon. This carboxyl group can have a nitrogen added (glutamine), it can be replaced by an amino group (ornithine in arginine synthesis), or it can be reduced to an aldehyde which is reactive (proline). • We already discussed glutamine synthesis. The -carboxyl group is activated by phosphorylation, and ammonia is added to form an amide linkage. 10. Essential and nonessential amino acids (what does this mean), difference between plants, bacteria and higher animals. Do not memorize the amino acids for this classification. • Essential Amino Acids- Amino acids that are obtained through diet because we are incapable of synthesizing them • Nonessential Amino Acids- amino acids that can be synthesized, not essential for diet • Higher animals don’t synthesize essential amino acids because their diets became adequate enough over time, and it would be superfluous to make them • Humans synthesize 10/20 • However, bacteria and plants synthesize their amino acids. 11. Similarites in the pathways of arginine and proline synthesis, cyclization and its prevention, mechanism of activation, biological reductant. • Proline synthesis follows a similar pattern (Fig 25.20). • The -carboxyl group is activated by phosphorylation. • The activated carboxyl is then reduced to an aldehyde. The resulting compound is called glutamate-5-semialdehyde. A semialdehyde has the properties of an aldehyde and a carboxylic acid. It is an aldehyde. • The aldehyde spontaneously interacts with the -amino group and cyclizes. • The resulting compound is reduced to give proline. • Both reductions require NADPH. • Arginine synthesis initially follows a similar pattern, at least as far as ornithine synthesis (Fig 25.21). • The first part of the pathway is the synthesis of ornithine from glutamate. • The first step of arginine synthesis is N-acylation (addition of an acetyl group to the - amino group of glutamate) to block the cyclization that occurs in proline synthesis. After that, the steps are similar to those in proline synthesis. • The -carboxyl group is activated by phosphorylation, and the activated carboxyl is reduced to an aldehyde. This would cyclize if the -amino group had not been blocked. • Now the aldehyde is free to react with something else, in this case, an amino group is added by an unusual aminotransferase. It should be noted that the nitrogen is not added to an -keto group, but to a terminal aldehyde. • The next step is removal of the acetyl blocking group, which generates ornithine. • The second part of the pathway is essentially addition of a urea (one C and 2 Ns) group to the nitrogen at the end of ornithine. This is relevant to the urea cycle. The urea is added in pieces, starting with one carbon and one nitrogen from carbamoyl-P (Fig 25.22). • Carbamoyl-P is unusual since it consumes two ATPs. CPS-I is a mitochondrial enzyme in higher organisms, probably because this is where the ATP is. It uses ammonia as a nitrogen source. E. coli has only one CPS, and it uses glutamine as a N source. The carbon source is bicarbonate (HCO ). • Ornithine transcarbamoylase adds carbamoyl-P to ornithine to form citrulline. This is also a mitochondrial enzyme. • The next steps replace an oxygen with a nitrogen. The oxygen of the ureido group is activated by AMP addition, and aspartate displaces the AMP. The resulting compound is cleaved, leaving behind the nitrogen of aspartate. Note that aspartate is a nitrogen donor here. Aspartate is a nitrogen donor in only three reactions: this one, and two in purine synthesis. 12. Carbamoyl-P synthetase, precursor for which compounds, most unusual aspect of reaction. How many enzymes in higher animals, and where are they located. • Precursor to citrulline, when carbamoyl is added to orthinine it makes citrulline • unusual since it consumes two ATPs • CPS-I is a mitochondrial enzyme in higher organisms, probably because this is where the ATP is. It uses ammonia as a nitrogen source. E. coli has only one CPS, and it uses glutamine as a N source. The carbon source is bicarbonate (HCO3). • Located in mitochondria 13. Final steps in arginine synthesis: common reactions with urea cycle. • The next steps replace an oxygen with a nitrogen. The oxygen of the ureido group is activated by AMP addition, and aspartate displaces the AMP. The resulting compound is cleaved, leaving behind the nitrogen of aspartate. Note that aspartate is a nitrogen donor here. Aspartate is a nitrogen donor in only three reactions: this one, and two in purine synthesis. 14. Urea cycle: which steps in mitochondria, its function, how nitrogen enters the cycle, what tissue has enzymes of the cycle. • This cycle was discovered by Hans Krebs before the citric acid cycle. Most of the steps are part of arginine synthesis. • It is a mechanism to remove nitrogen. The nitrogen can be ammonia (generated from a catabolic GDH, which will deaminate glutamate, which is generated from aminotransferases) and aspartate. The ammonia and bicarbonate are incorporated into carbamoyl-P. • The additional step that is not part of arginine synthesis is catalyzed by arginase, which converts arginine to ornithine and urea. • Elevated amino acid degradation increases glutamate, ammonia, aspartate (via an aminotransferase), and N-acetylglutamate. The latter compound stimulates CPS-I activity. • It should be noted that this is an energy-intensive process. • The process is confined to the liver. 15. Asparate family: similarities with glutamate (α-ketoglutarate family), asparagine synthesis (mechanism of activation), control of first reaction of common pathway • Like glutamate family amino acids, almost all the chemistry is at the terminal carboxyl group, and this requires activation of the carboxyl group by similar mechanisms. • Asparagine is synthesized in a reaction similar to the GS reaction (Fig 25.26). The carboxyl group is activated with ATP forming aspartyladenylate. • For all of these amino acids, aspartate is first phosphorylated on the carboxyl group, to form aspartyl phosphate. 16. Methionine synthesis, how sulfur is added, vitamins and cofactors involved, mechanisms that result in accumulation of homocysteine, methyl group addition, transsulfuration pathway. • Methionine from homoserine (Fig 25.27). Methionine differs from homoserine in that it has an SH instead of an OH, and a methyl group is added to the SH. • The first three steps of methionine synthesis are simply replacing the OH with an SH. • First, the OH is activated by succinylation with succinyl-CoA. • Then cysteine, the universal sulfur donor in bacteria, replaces the succinyl-CoA. • The cysteine is then removed, except for the SH which remains behind. This produces a compound called homocysteine, this is one carbon longer than cysteine. • The last step is addition of a methyl group using a THF derivative, N5-methyl-THF. • Methionine has several important functions. • The first amino acid for proteins. • In proteins, the S group can be oxidized, and methionine residues surround and protect active sites from oxidative damage. • The third function is as a precursor for S-adenosylmethionine (SAM), a methyl donor (Fig 25.28). SAM can also be a donor of propylamino groups. Note that the methyl group comes from a THF derivative. • Homocysteine and heart disease (Fig.) • Elevated homocysteine in the blood may be an independent risk factor for heart disease. • There are two forms of homocysteinuria: • One results in elevated homocysteine and methionine. This one results from a block in the conversion of homocysteine to cysteine. It is easily cured by vitamin B6 therapy. • A second results from high homocysteine, but normal methionine. This is because of a block in converting homocysteine to methionine. It is cured with vitamin B12 therapy. • Folic acid (the precursor for THF) therapy could also help. • Both forms of homocysteinuria result in extraordinary cardiovascular damage. It is thought that homocysteine, S-adenosylhomocysteine, or a compound derived from homocysteine is destroying the arteries. 17. Pyruvate family: alanine synthesis, precursors for branched chain amino acids, role of thiamine pyrophosphate and of shared enzymes, leucine synthesis pathway mimicks what pathway. • Family members are alanine, valine, leucine. Isoleucine is a member of the aspartate and pyruvate family. • Alanine is synthesized by a glutamate-alanine transaminase in all organisms. • The next reaction for both isoleucine and valine synthesis is addition of a two-carbon fragment from hydroxyethyl-thiamine pyrophosphate (TPP), with pyruvate providing the carbon. Other TPP-containing enzymes include pyruvate DH, and transketolase. • Leucine synthesis mimicks the first reactions of the citric acid cycle • -ketobutyrate, -keto acid, and pyruvate are precursors for branched chain amino acids 18. 3-phosphoglycerate family: serine, glycine, and cysteine synthesis; synthesis of C1 intermediates, sulfur assimilation (what compound is environmental sulfur source, what is actually incorporated into amino acids). What is the major donor for sulfur in various biosyntheses. • These amino acids are derived from 3-phosphoglycerate, a glycolytic intermediate. • Serine (Fig 25.31) synthesis starts from oxidation (NAD as electron acceptor) of 3-PG to an -keto acid. The -keto acid is transaminated to form 3-phosphoserine, and dephosphorylated to generate serine. Serine inhibits the first enzyme of the pathway. • Glycine (two carbons) is made from serine (three carbons) in one step (Fig 25.32, top only). The extra carbon from serine is donated to the C1 carrier, THF to form N5, N10- methylene-THF. This reaction, serine hydroxylmethyltransferase, requires pyridoxal phosphate. • The text also mentions the synthesis of glycine by a reversal of the “glycine oxidase” reaction. I am not familiar with this reverse reaction. Glycine can be cleaved by the “glycine cleavage enzyme” to produce CO2, NH3, NADH, and N5, N10-methylene-THF. This reaction can be called glycine oxidase, and is used to generate N5, N10-methylene-THF. Note that the reverse reaction requires NADH, which should not be favorable. • Cysteine synthesis proceeds from serine, usually in two steps in bacteria (Fig 25.33). The reaction mechanism involves replacement of an OH with an SH group. This is accomplished by first activating the hydroxyl group by acetylation with acetyl-CoA. This reaction, the first committed step in cysteine synthesis, is inhibited by cysteine. The next step is sulfhydrylation by H2S, which replaces the acetyl group. The sequence of reactions is the primary mechanism of sulfur assimilation. Cysteine is the major donor of sulfur for reactions in bacteria. It is more complicated in higher organisms, where methionine can provide the sulfur. • Sulfate assimilation (Fig 25.34). Sulfur in the environment is available as an oxidized compound, sulfate. H2S is toxic, and is rapidly consumed by bacteria, as a reduced compound that can be used for energy generation. Sulfate is reduced after activation of the sulfate by ATP to form APS and PAPS. Thioredoxin is then used as a reductant to reduce sulfate to sulfite, and then NADPH reduces sulfite to sulfide. 19. Aromatic family: precursors, endproduct of common pathway, regulation of first reaction, (can ignore tryptophan synthesis, except know which compounds are required for it synthesis), conversion of phenylalanine and tyrosine, and disease that results from problems with the interconversion. • Precursors – chorismate • Chorismate is the endproduct • The first step of the common pathway is regulated in a fashion similar to that of the common pathway of the aspartate family. There are three enzymes that catalyze the first step in E. coli, and each of the amino acid endproducts inhibits and represses one of the enzymes. The inhibition is only partial because amino acids are not the only compounds made from these pathways. • Phenylalanine and tyrosine (Fig 25.37). Both amino acids have prephenate as an intermediate. • For both amino acids, CO2 is removed from chorismate, and for phenylalanine, an OH is removed. • In both cases, the next step is a transamination with glutamate as the nitrogen donor. • The two amino acids partially inhibit the common pathway at the first step, and they also inhibit the first committed steps, i.e., the synthesis of prephenate. • Some organisms can synthesize tyrosine from phenylalanine (Fig 25.38). This requires a hydroxylation with O2 as the oxygen donor, tetrahydrobiopterin (a THF derivative), and NADPH to reduce the oxygen to the OH level. Tyrosine is classified an a nonessential amino acid, as long as phenylalanine is provided in the diet. Phenylalanine is essential. • Some individuals lack the monooxygenase that catalyzes phenylalanine hydroxylation. Dietary phenylalanine is not converted to tyrosine, and is instead deaminated. This results in accumulation of phenylpyruvate in the urine, phenylketonuria. Mental retardation and other neurological disorders develop without dietary intervention (a low phenylalanine diet). • Synthesis of Tryptophan requires phosphoribosyl-PP (PRPP) and serine, both of which provide carbons 20. Histidine synthesis: precursors, role of ATP • Histidine (Fig. 25.40) is in a family of its own. It is an unusual pathway which requires PRPP and ATP as carbon precursors. The fifth step is unusual in that it results in ring closure, which is required for formation of the imidazole ring of histidine, and release of an intermediate in purine synthesis from the partially altered ATP. Histidine inhibits the first reaction of the pathway, and represses the synthesis of all enzymes in E. coli. 21. The logic of herbicides (what type of pathways targeted), but knowledge of specific herbicides is not required. • Herbicides (weed killers) and amino acid analogs. Plants can synthesize all 20 amino acids. Inhibitors that target precursors of our essential amino acids should not be toxic to us. 22. Amino acid degradation: what is difference between glycogenic and ketogenic amino acids, how nitrogen is removed, and where does it go, alkaptonuria and how are symptoms prevented. • The glycogenic amino acids are those that can be converted to glucose. Those degraded to acetate or acetoacetate are ketogenic, and can be used to synthesize fatty acids or ketone bodies. • Nitrogen removal. The carbon skeletons of the amino acids can be degraded. An early step in such catabolism is removal of the nitrogen. • The first step is often loss of the nitrogen by transamination, with -KG as the acceptor, which generates glutamate. The nitrogen of glutamate can be oxidatively deaminated by glutamate dehydrogenase, which generates -KG and ammonia (Fig 25.4 from Zubay). NAD is the cofactor. The ammonia can enter the urea cycle in the formation of carbamoyl-P. This GDH is mitochondrial, like carbamoyl-P synthetase. The synthetic GDH is cytosolic. • Another disease of amino acid metabolism is alkaptonuria. It is caused by a block in one enzyme of tyrosine catabolism. It results in darkening of the cartilaginous tissues, and urine that turns black on exposure to air. Feeding of phenylalanine results in increased secretions of homogentisic acid. A London pediatrician in 1908 proposed a defective enzyme, and that the disease was inherited as a Mendelian recessive. In other words, he was proposing that genes code for proteins. The 1958 Nobel Prize was awarded to Beadle and Tatum who proposed the one gene-one enzyme hypothesis. They noted that their hypothesis was implicit in Garrod’s work. 23. Structures to be able to recognize: first intermediate in pathways of synthesis of proline (glutamyl-P), arginine (N-acetylglutamate), asparagine (aspartate), lysine-threonine- methionine common pathway (aspartyl-P), methionine (homoserine), alanine (pyruvate), serine (3-phosphohydroxypyruvate), cysteine (O-acetylserine), glycine (serine), and histidine (N1-5’-phosphoribosyl-ATP). 24. The last intermediate in synthesis of proline (1-pyrroline-1-carboxylate), arginine (argininosuccinate), methionine (homocysteine), serine (3-phosphoserine), phenylalanine (phenylpyruvate), tyrosine (4-hydroxyphenylpyruvate), tryptophan (indole), and histidine (histidinol). [Show More]
Last updated: 1 year ago
Preview 1 out of 32 pages
Instant download

Buy this document to get the full access instantly
Instant Download Access after purchase
Add to cartInstant download
Also available in bundle (1)

BIOL 3362 - Exam 1 -4 Study Guide BUNDLE | Download To Score A.
BIOL 3362 - Exam 1 -4 Study Guide BUNDLE | Download To Score A.
By A+ Solutions 2 years ago
$14
5
Reviews( 0 )
Document information
Connected school, study & course
About the document
Uploaded On
Sep 22, 2021
Number of pages
32
Written in
Additional information
This document has been written for:
Uploaded
Sep 22, 2021
Downloads
0
Views
39