*NURSING > STUDY GUIDE > NR 511 Week 8 Final Exam (Study Guide) | Download To Score An A (All)
NR 511 Week 8 Final Exam (Study Guide) | Download To Score An A
Document Content and Description Below
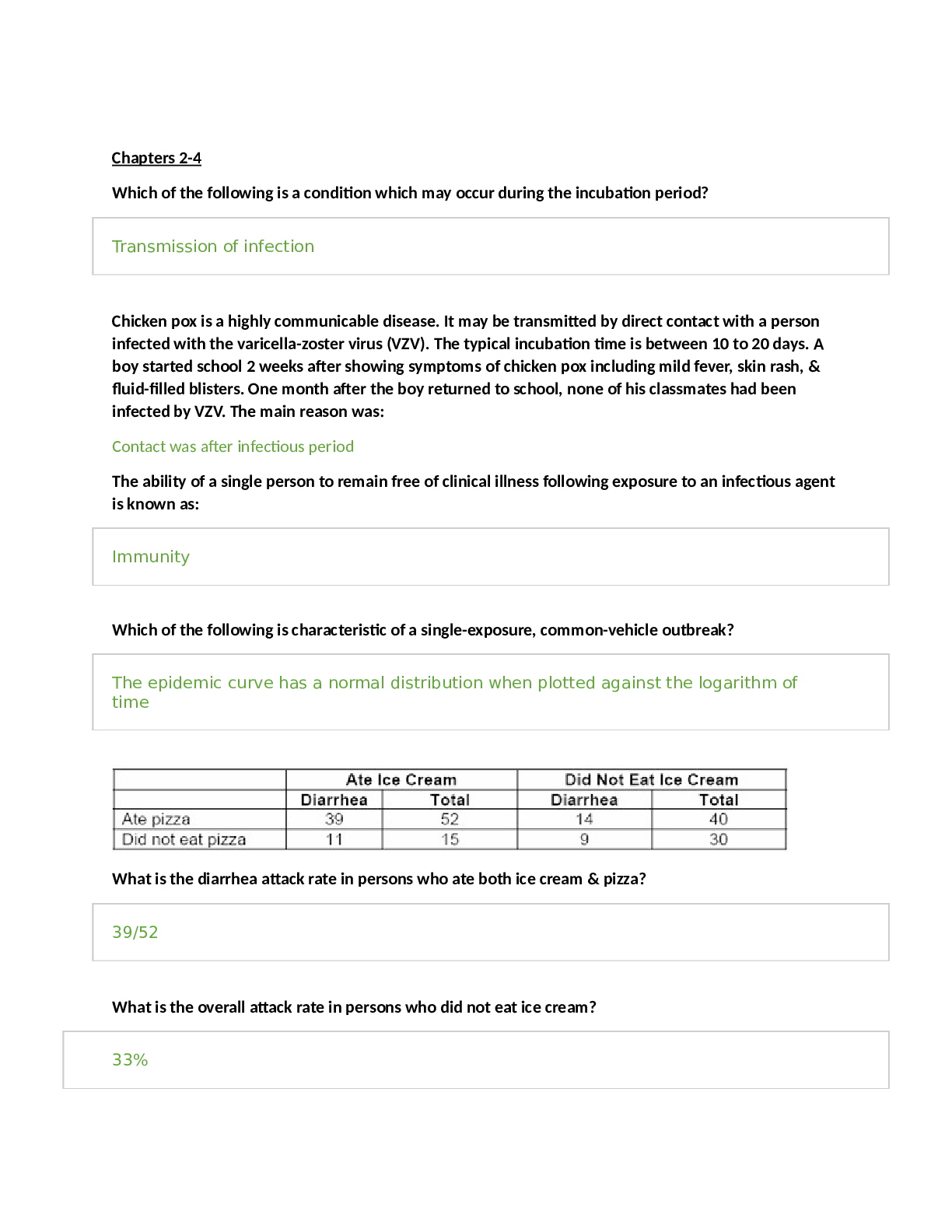
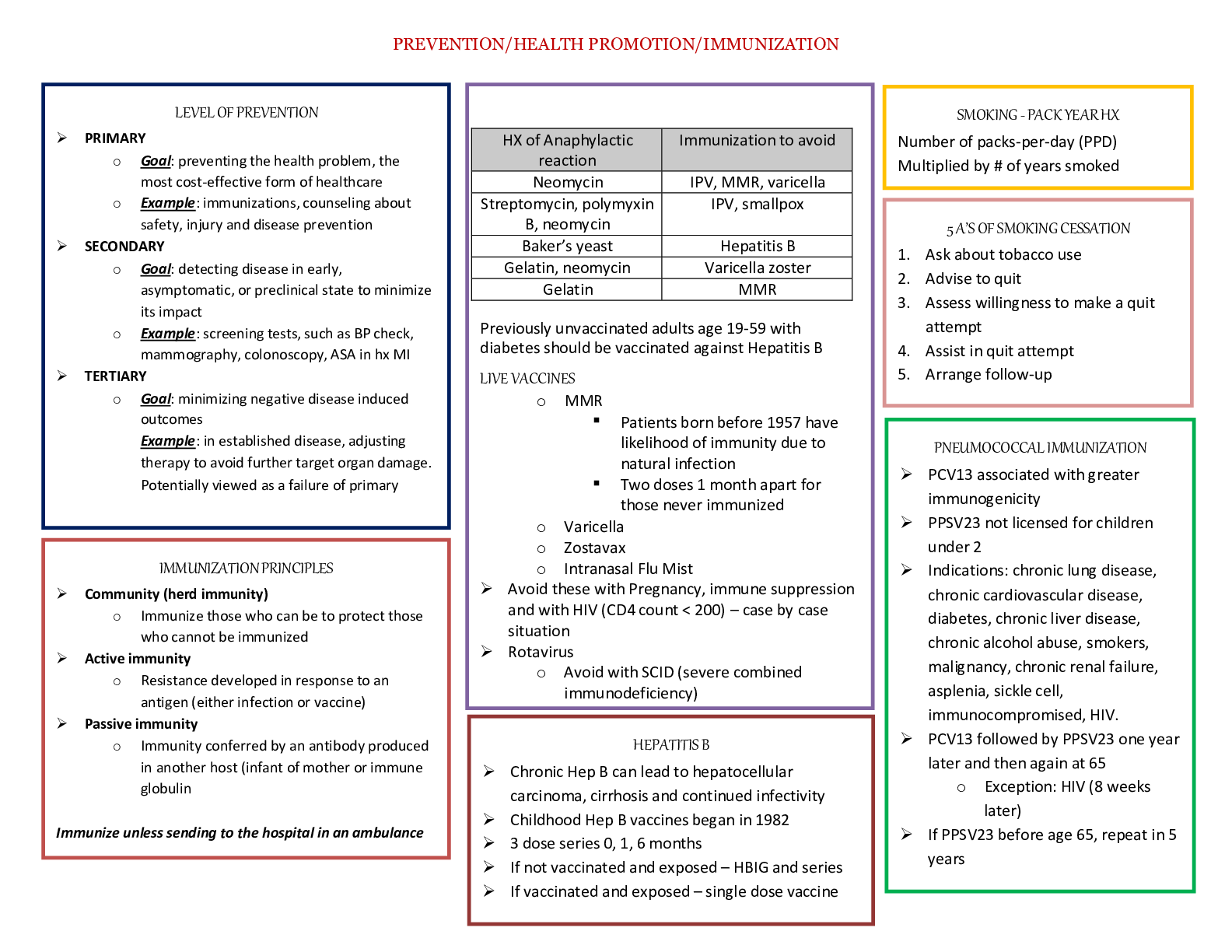
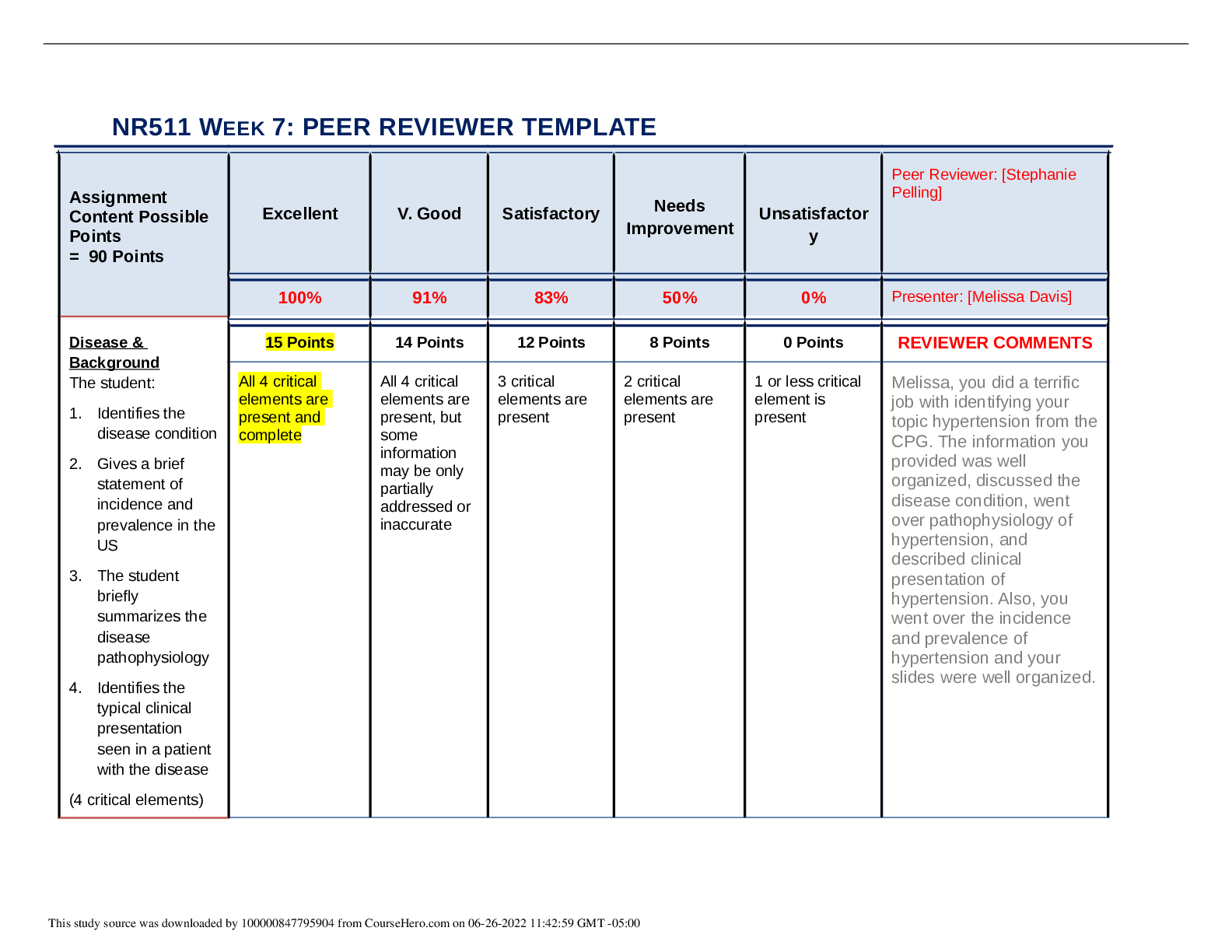
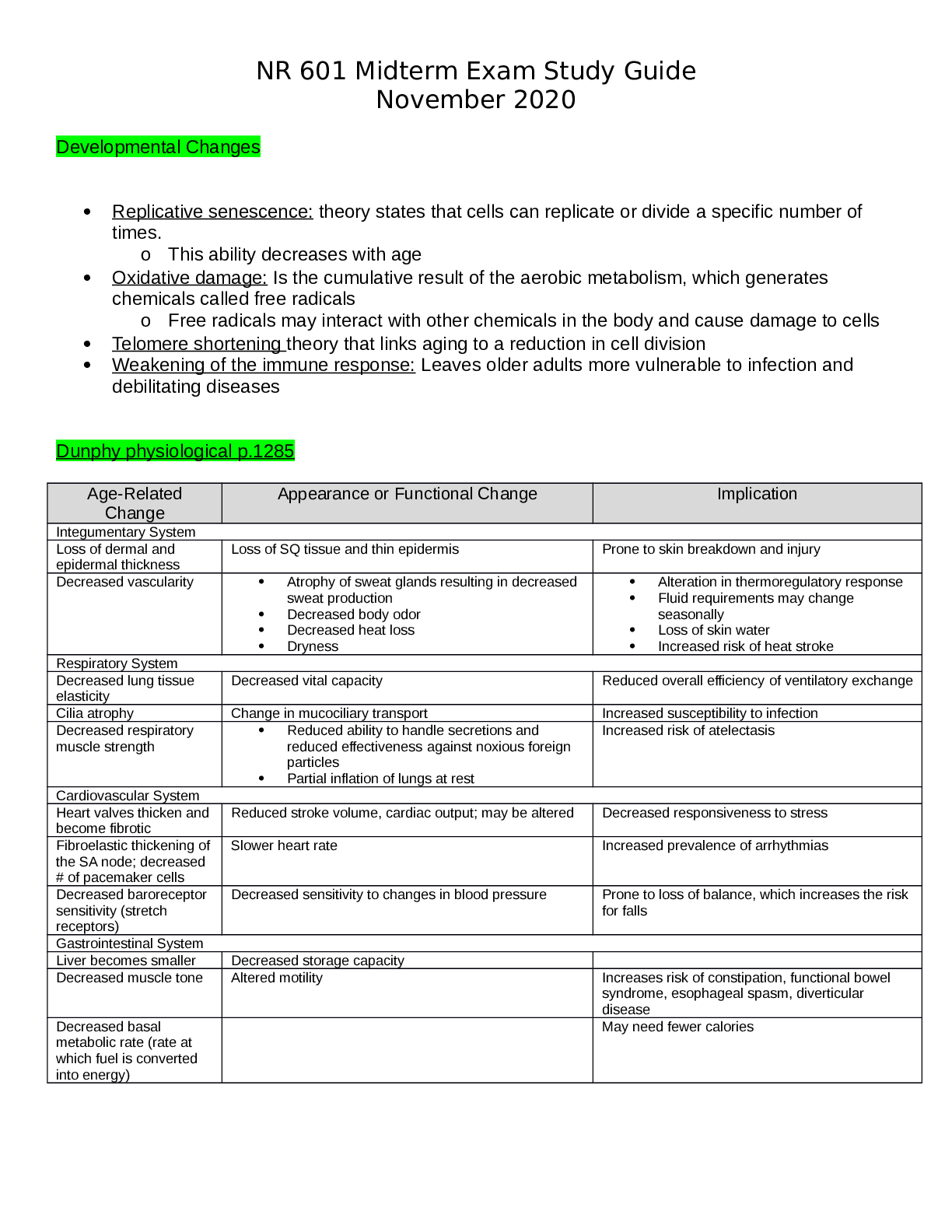
Topics: o Common M/S disorders o Common spine disorders o Metabolic disorders o Endocrine disorders o Wounds, lacerations & bites o Common hematological disorders o Common male GU disorders ... o Testicular disorders Chapters + lectures: Wk 5: o Chapter 52: Common Musculoskeletal Complaints o Chapter 53: Spinal Disorders o Chapter 54: Soft-Tissue Disorders o Lectures o Hollier o DE Wk6: o Chapter 57: Glandular Disorders (p. 880-897 only) o Chapter 59: Metabolic Disorders o Chapter 73: Common Injuries (p.1210-1223 only) 1212-1213 table 73.1 o Review thyroid lecture again o Lectures o Hollier o DE Wk7: o Chapter 46: Nocturia in Men (p. 682) & Testicular Pain (p. 685) o Chapter 49: Prostate Disorders o Chapter 50: Penile & Testicular Disorders o Chapter 61: Hematological Disorders o Lectures o Hollier o DE Completion of study guide: IIII 1. Signs and symptoms and management of musculoskeletal sprains/strains/dislocations Signs and symptoms and management of musculoskeletal sprains/strains/dislocations Sprains: stretching or tearing of ligaments that occurs when a joint is forced beyond its normal anatomical range First degree- stretching of ligamentous fibers Second degree- partial tear of part of the ligament with pain and swelling Third degree- complete ligamentous separation Sprain- sudden injury or fall that caused acute pain and swelling that got worse over a few hours, redness and bruising, active and passive ROM decreased. Radiography to rule out fx. Strain: muscle injury caused by excessive tensile stress placed on a muscle that results in stiffness and decreased function -effects muscle or tendon that connects a muscle to a bone, complain of “pulled muscle,” severe cases cause inflammation, swelling, weakness and loss of function-surgery may be needed Management: PRICE (protect, rest, ice, compression, elevation), limitation of activity, physical therapy, NSAIDS, referral to ortho Dislocation- complete separation of 2 bones that form a joint Very painful and cause immobility, need immediate medical attention Referral to orthopedics for possible surgery or reduction with application of cast or splint. four cardinal signs of inflammation (erythema, warmth, pain, or swelling) -SPEW 2. Signs and symptoms and management of spinal disorders (spondylosis, stenosis, etc.) Cervical Spondylosis- neck stiffness, mild aching discomfort with activity. Pain and limited ROM occur with lateral rotation and lateral flexion of the neck toward the affected side. Weakness shoulder abduction- C5. Bicep weakness- C6. Tricep weakness-C7.Myelopathy- leg weakness, gait disturbance, balance problems, difficulty performing fine motor tasks, loss of bowel and bladder. Treatment- cervical traction, PT, pain relievers. Surgery for Myelopathy. Low back pain-Tenderness and decreased range of motion. Positive straight leg test. Treatment- NSAIDS, muscle relaxants, opioids, surgical, self-care, spinal manipulation Stenosis-pseudoclaudication causing radicular pain in the calves, buttocks, and upper thighs of one or both legs. Symptoms progress from a proximal to distal direction. Walking or prolonged standing causes pain and weakness in buttocks and legs. Stooping over helps relieve pain. Positive Romberg. Reflexes diminished. With bowel or bladder symptoms, sphincter tone may be decreased Management- surgical decompression. NSAIDS, folic acid, vitamin b12. PT-flexing the spine.Bicycling. Intermittent use of NSAIDs may be helpful, as well as folic acid or vitamin B12 supplementation in some cases depending on results of laboratory tests. Management revolves around physical therapy or an exercise program that focuses on flexing the spine. Flexion of the spine increases intraspinal volume. Bicycling is one exercise that is done with the spine in flexion. Improving abdominal muscle tone lifts the pelvis anteriorly and flexes the lumbar spine. Reduction of intra-abdominal fat is critical to achieving the objective. Thus, weight loss may be pivotal. Lumbar flexion exercises increase spinal canal volume. Examples include exercise on all fours, arching the back, or in the fetal position. Exercises that extend the spine should be avoided (swayback). 3. Recognition and immediate management of cauda equina syndrome Immediate management of cauda equina syndrome. (P. 829) Cauda equina syndrome is a medical EMERGENCY and requires immediate decompression. If Cauda equina is confirmed, surgical lumbar decompression is necessary to halt neurological deterioration unless surgery is contraindicated for other medical reasons. *Rational on Davis Edge question: Low back pain accompanied by acute onset of urinary retention or overflow incontinence, loss of anal sphincter tone or fecal incontinence, loss of sensation in the buttocks and perineum, and motor weakness in the lower extremities is a red flag for cauda equina syndrome or severe neurologic compromise. Perianal numbness. Cauda equina compression is characterized by bilateral lower extremity weakness, anesthesia, or paresthesia of the perineum and buttocks (saddle anesthesia). There may or may not be bowel or bladder incontinence or bladder retention. When there is neurologic deficit affecting the bowel or bladder, these changes may not be reversed with surgical decompression, 4. Maneuvers and expected findings with joint pain (knee, shoulder, wrist, etc.) Neck pain-Spurling’s. Shoulder pain-Apley scratch test(reaching the scapula). Internal and external flexion. Internal and external abduction. Pain with abduction= early supraspinatus tendinitis and subacromial bursitis=early rotator cuff injuries. Wrist and hand-allen’s test= radial and ulnar arteries. Phalens test=median nerve compression. Tinel’s sign assess for compression neuropathy – tapping over nerve. Finkelsteins test- de Quervains disease. Thumb between finger and point. Knee Pain= Mcmurray, apprehension sign, bulge sign, inspect/palpate to assess effusion. Lachman, drawer sign – ACL, Thumb test - PCL MCL, LCL test are valgus and varus Tennis elbow cup coffee cup sign CTS-NSAIDs not effective Achillies rupture – Thompson test 5. Initial assessment of FOOSH injury in correlation to anatomical location of radial head bone Lisa Callahan FOOSH - Falling On an Out Stretched Hand. After falling on an outstretched hand patients present after trauma with pain and swelling in the distal forearm or wrist. Numbness may be present if the medial nerve is affected. The mechanism of injury will often provide important clues to the diagnosis. The examination begins with gentle palpation to locate the area of point tenderness and includes a thorough neurovascular assessment. A radiograph of the wrist (including an oblique view) may be necessary to rule out fracture. Common fractures are the Colles fracture of the distal radius and the navicular (scaphoid) fracture of the anatomical snuffbox. It is not unusual to have a navicular fracture missed on radiography, so an orthopedic referral should be provided when the presenting complaint is pain and trauma to the soft-tissue area of the anatomical snuffbox. Scaphoid injury. 6. Assessment and management of Myofascial pain Trigger points within a muscle. Common cause of nonarticular rheumatic pain. Injections at the trigger point with saline, an anesthetic, or corticosteroid, dry needling, muscle relaxant tizanidine, NSAIDS, or cyclooxygenases-2 inhibitors. Tricyclic antidepressants. 7. Health promotion activities to prevent sport related musculoskeletal injuries Protection may refer to preventing the injury from occurring or making it less severe by wearing protective gear, such as helmets, wrist pads, and kneepads. Maintain adequate hydration and proper diet while playing sports. Stretch before the activity. Stop when you are injured, do not “tough it out”. 8. Osteopenia Osteopenia: • Osteopenia Is the precursor to osteoporosis. Osteopenia is categorized by the level of T- scores in relation to the results of a dual-energy x-ray absorptiometry scan or (DXA Scan), which measures the mineral content of bone. A T-score ranging from -1 to -2.5 would be classified as osteopenia. Pathophysiology: • It occurs secondary to uncoupling of osteoclast-osteoblast activity, resulting in a quantitative decrease in bone mass. Peak bone mass is typically achieved by males and females just prior to, or early-on in the 3rd decade of life. • Beyond age 30, bone resorption gradually becomes favored as dynamic bone remodeling continues into later decades of life. • Histologic specimens demonstrate markedly thinned trabeculae, decreased osteon size, and enlarged haversian and marrow spaces. Osteopenia Prevention: • Certain habits can accelerate the process such as: o Smoking – prevents calcium uptake o Not getting enough calcium and vitamin D o Drinking too much ETOH o Use of certain medications (i.e.: corticosteroids and anticonvulsants) o Not getting enough weight-bearing exercise (at least 30 mins on most days). If your feet touch the ground during an exercise, it’s probably weight bearing. Running and walking are weight bearing. Swimming and biking are not – non-weight bearing o Falls • Women are more likely to have low bone density than men, but it’s no longer viewed as solely a women’s condition. • Approx. a third of white and Asian men over age 50 are affected. • Percentages for Hispanics (23%) and blacks (19%) are lower, but still sizable. Current National Osteoporosis Foundation (NOF) recommends testing for: • Women 65 and older • Postmenopausal women younger than 65 who have one or more risk factors, which include being thin • Postmenopausal women who have had a fracture • For men: testing is done more on a case-by-case basis. Osteopenia Treatment: Can be treated with exercise and nutrition or with medications. • If T-score is under -2, need to ensure you are doing regular weight-bearing exercise, and getting enough vitamin D and dietary calcium. • If T-score is closer to -2.5, a medication may be considered to keep bones strong. • Bisphosphonates are most commonly prescribed medication class for treatment to prevent/reduce reabsorption of bone. Prolonged use has been linked with 2 major clinical side effects: osteonecrosis of the jaw (ONJ) and the atypical subtrochanteric femur fracture. DE quiz question. • ONJ is rare and is associated with IV forms and not oral forms of the medication. Tx entails immediately stopping the offending agent. • Atypical femur fractures also are rare but have significant associated morbidity, and clinicians are cautioned against the chronic, uninterrupted bisphosphonate use beyond 3 to 5 years or in situations when pts report mild thigh discomfort while undergoing tx. • The core treatment options for osteopenic patients involve early education on how to achieve and maintain healthy bone mass levels and extensive education and counseling on the relevant social, environmental, and lifestyle risk factors that compromise bone health. General consensus favors pharmacologic treatment in a patient with spine or hip fractures in addition to a documented low BMD. Treatment recommendations vary for other nonvertebral fractures and include the following: • The National Osteoporosis Society (NOS) recommends starting treatment in all postmenopausal women with a history of any fragility fracture • The National Osteoporosis Foundation (NOF) recommends performing DXA scans on patients sustaining nonvertebral fragility fractures, and the decision to treat or not with pharmacotherapy is based on the patient’s t-score; patients considered to be osteopenic (t- score between -1 and -2.5) are not started on drugs. Pharmacotherapy agents work through either anti-resorptive or anabolic means. Bisphosphonates are the most commonly prescribed medication class. These drugs are divided into non-nitrogen and nitrogen-containing compounds. The latter are considered first-line therapy. The nitrogen-containing compounds inhibit farnesyl pyrophosphate synthase and ultimately inhibit osteoclast resorption and induce osteocyte apoptosis. Common agents include: • Alendronate may reduce the rate of hip, spine, and wrist fractures by 50% • Risedronate may reduce vertebral and nonvertebral fractures by 40% over three years • IV zoledronic acid reduces the rate of spine fractures by 70% and hip fractures by 40% over three years Other Medication Classes • Conjugated estrogen-progestin hormone replacement (HRT) • Estrogen-only replacement (ERT) • Salmon calcitonin (Miacalcin, Fortical) • Selective estrogen receptor modulators (Raloxifene) - Raloxifene is an agonist to estrogen receptors on bone and reduces osteoclast resorption • Anabolic (Teriparatide) - Teriparatide is a recombinant form of parathyroid hormone (PTH) that stimulates osteoblasts to produce more bone. Teriparatide is now FDA approved for osteoporosis treatment in males and females • RANKL inhibitors (Denosumab) - Denosumab is a monoclonal Ig2 that targets RANKL and inhibits its ability to bind to RANK and results in the inhibition of osteoclast activation • The t-score is measured in standard deviations and reflects the difference between the patient's measured BMD and the mean value of BMD in healthy, young, matched controls (30- year-old women). By definition, a normal BMD measurement is within one standard deviation of the young adult mean. The WHO defines t-scores between -1 and -2.5 as osteopenic and scores below -2.5 as osteoporotic. Greater than -2.5 means osteoporosis. The z-score is also measured in standard deviations, but the z-score is compared to a healthy, age-matched control group. The z-score is most clinically relevant when obtaining a DXA scan in younger patients when secondary osteoporosis is being considered. A z-score less than -1.5 warrants a comprehensive secondary osteoporosis workup. • Standard laboratory workup includes checking calcium, phosphorus, albumin, alkaline phosphatase, liver function tests, creatinine (serum and urine), 25 hydroxyvitamin D, TSH and free T4, and intact PTH levels. Males should have a free testosterone level checked to rule out hypogonadism. • The WHO created a fracture risk assessment tool (FRAX score) to predict the 10-year risk of sustaining a hip or other major osteoporotic fracture. These other major fragility fractures include fractures of the spine, wrist, forearm, or humerus. The assessment includes 12 questions weighted by the relative risk associated with a future fragility fracture event. Assessment includes age, sex, personal history of fracture, low BMI, oral steroid use, secondary osteoporosis, parental history of hip fracture, smoking status and alcohol intake. In addition, optional BMD measurement values can be included from a prior DXA scan (if available) to provide a more comprehensive score report. Obesity is a predisposing factor, as is osteoporosis 9. Assessment and management of gout Persons from the United States, the Pacific Islands, and countries with abundant lifestyles have an increased incidence of gout. Gout is more prevalent in African American men. The increased incidence of gout in older adults has been associated with an increased use of diuretics – HCTZ DEQ. Patients with gout may experience an acute attack with rapid fluctuations of serum urate levels. Surgery, dehydration, binge alcohol consumption, emotional stress, infections, diuretics, and uricosuric drugs can all cause rapid fluctuations in serum urate levels. Causes of primary gout include idiopathic inborn errors of purine metabolism, decreased renal clearance of uric acid, and specific enzymatic defects such those resulting in Lesch-Nyhan syndrome and glycogen storage disease. Secondary causes of gout include other disease processes and medications, such as thiazide diuretics, that result in an overproduction or underexcretion of uric acid. The patient will present during an acute attack with pain, tenderness, erythema, and swelling of the affected joints. the joint most frequently affected is the first metatarsophalangeal joint of the great (big) toe; however, the midfoot, knees, fingers, wrists, and elbows may also be affected. The typical presentation is excruciating pain that awakens the patient at night. Patients often describe the pain as throbbing, crushing, and pulsating. The pain is not relieved by rest or positional changes and prevents weight-bearing on the affected limb. Often the patient cannot tolerate anything coming in contact with the affected joint—even bed clothing touching the limb can be extremely painful. The clinical presentation and medical history findings are often sufficient to diagnose gout. A definitive diagnosis is only made with identification of sodium urate crystals in the aspirated fluid from affected joints. The goals of clinical management are to terminate an acute attack, prevent future attacks, normalize hyperuricemia, and prevent potential complications of urate deposits. Management of gout includes pharmacological treatment of acute attacks and long- term medical and pharmacological treatment of hyperuricemia. Acute management of gout includes generalized rest, elevation and immobilization of affected joints, and pharmacological treatment. The initial medication of choice for acute gout attacks is an NSAID. Colchicine is an effective medication to terminate an acute attack of gout if administered within 36 hours of the initial onset of symptoms. Corticosteroids can provide dramatic systematic relief and can be administered orally, intramuscularly, or intra-articularly. The long-term management of gout includes pharmacological agents, dietary modifications, activity evaluation, and education regarding the prevention of gout. Patients with extensive or large tophi may benefit from surgical excision of these lesions. Physical activity must be restricted during an acute gout attack, and bedrest should be maintained for 24 hours following an acute attack. The joint should be immobilized; if a lower extremity is involved, no weight-bearing should be allowed during the acute attack. During intercritical periods, physical therapy may be indicated to maintain or improve function. Hot compresses may promote comfort after an acute attack but should not be instituted until the acute pain subsides, usually 24 to 72 hours after the initiation of therapy. The patient should apply heat for 20 minutes two to three times daily through the use of moist heating pads, warm showers and baths, or moist towels heated in a microwave. Relief may also be obtained using ice packs during an acute attack. Patients should be instructed to apply packs for only 10 to 20 minutes sessions at a time to avoid thermal damage to the skin; ice packs should be discontinued if pain is not relieved. Long-term management includes dietary moderation of purine-containing foods (limited to no more than one to two servings of purine- rich foods per day), moderating alcohol intake, maintaining weight, and sufficient physical activity to maintain joint mobility during quiescent periods between gout flares. 10. Medication management for acute vs. chronic gout Acute-rest, elevation and immobilization. NSAIDS, colchicine (onset of symptoms less than 36 hours), and corticosteroids. Avoid aspirin. Avoid excessive alcohol. Avoid purine-rich foods. Chronic- uric acid secretion <1,000 mg/24 hrs: probenecid. Uric acid secretion >1,000mg/24 hrs: allopurinol. Colchcine. 11. Dietary restrictions for gout Restrict purine foods, (Examples • All meats and seafoods (especially organ meats such as liver, kidneys, and sweetbreads [thymus, pancreas]) • Meat extracts and gravies • Yeast and yeast extracts (brewer’s and baker’s) • Beer and alcoholic beverages • Beans, peas, lentils, oatmeal, spinach, asparagus, cauliflower, and mushrooms • Mussels and scallops • Anchovies, herring, and sardines • Trout, haddock, mackerel, and tuna 12. Signs and symptoms Hyperthyroidism: inverse (low TSH, High T4) and management of thyroid disorders • excessive secretion and synthesis of one or both of the thyroid hormones thyroxine (T4) and triiodothyronine (T3) • Risk factors: Women age 20-40 • Causes: • Graves’ disease is the most common cause of spontaneous hyperthyroidism. It is an autoimmune disorder characterized by autoreactive, agonistic antibodies to the TSH receptor • Graves’ disease accounts for 80% to 90% of hyperthyroid cases • Subacute thyroiditis is the most common cause of thyrotoxicosis, accounting for 15% to 20% of cases. Characterized by glandular inflammation and follicular cell destruction, viral etiology, frequently occurring following an acute viral infection. • Toxic multinodular goiter (Plummer disease) is as common as subacute thyroiditis, more common in other parts of the world where dietary iodine deficiency is prevalent. • A tumor of the pituitary gland causing hypersecretion of TSH (thyrotropin) is a rare cause of hyperthyroidism • S/S: Anxiety, nervousness, diaphoresis, fatigue, heat intolerance, palpitations, weight loss, and insomnia. In situations in which the thyroid tissue has become enlarged, the patient may complain of fullness or pressure in the neck. Additional symptoms include weakness, exercise intolerance, tremors, lower extremity edema, weight loss in the presence of an increased appetite, menstrual irregularities, frequent bowel movements or diarrhea, and exertional dyspnea. Eye complaints include blurred vision, proptosis (downward displacement of the eyeball), photophobia, and double vision. Patients may also report that they are unable to concentrate, extremely irritable, and emotionally labile • clinical manifestations of thyrotoxicosis, which include palpitations, diaphoresis, heat intolerance, and anxiety • Testing: sensitive serum TSH assay, measurement of T4 and T3 levels, Graves’ disease, antithyroglobulin and antimicrosomal antibodies are elevated A 24-hour radioactive iodine uptake (RAIU) test can differentiate Graves’ disease from subacute thyroiditis and toxic nodular goiters, thyroid ultrasound -subabute thyroiditis – low uptake - Graves – rapid uptake • Treatment: beta blocker initially, radioactive iodine therapy, Two antithyroid drugs are used—propylthiouracil (PTU) and methimazole (MMI), ablation or surgery Hypothyroidism: • Primary: 95% of patients with hypothyroidism, dysfunction or atrophy of the thyroid gland • Central: thyroid dysfunction is caused by failure of the pituitary gland, the hypothalamus, or both, • Secondary: failure of the pituitary gland to secrete adequate amounts of TSH • Tertiary hypothyroidism: inadequate secretion of thyrotropin-releasing hormone (TRH) by the hypothalamus or failure of TRH to activate its cognate receptors within the pituitary gland • Causes: atrophy (probably autoimmune), TSH receptor–blocking antibodies, Chronic autoimmune thyroiditis (Hashimoto’s disease) Amiodarone, External radiation Status—post- radioiodine (131I) treatment Status—post-thyroidectomy Infiltrating disorders: malignancy, granulomatous disease, Thyroid dysgenesis • S/S: early symptoms are often subtle and nonspecific, fatigue, dry skin, slight weight gain, cold intolerance, constipation, and heavy menses. Myalgia, muscle cramps, headaches, and weakness, very dry skin, coarse hair, loss of lateral eyebrows, alopecia, hoarseness, continued weight gain, slight impairment in mental ability, depression, decreased libido, decreased GI motilitiy and hypersomnia. patients with autoimmune thyroid disease and atrophic gastritis who also present with pernicious anemia • Testing: measuring serum TSH. TSH and FT4, antithyroid antibody titers—either for antimicrosomal (anti-TPO) antibodies or antithyroglobulin antibodies • Side note: Medications such as metoclopramide (Reglan) increase TSH levels. Dopamine (Intropin), glucocorticoids, NSAIDs, and somatostatin decrease TSH levels. Other medications, such as phenytoin (Dilantin), amiodarone (Cordarone), and lithium carbonate, can also affect thyroid function tests. Smoking (nicotine) also impacts thyroid hormone levels. These medication and chemical exposures act via a number of mechanisms to affect thyroid function. A drug may bind with albumin and displace thyroid hormone off carrier proteins, or it may prevent albumin from binding with T3 or T4, in each case resulting in more active hormone in circulation. Some drugs may cause an upregulation in metabolic processing proteins (i.e., different cytochrome P oxidase isomers), which normally inactivate thyroid hormones; thus, their upregulation can lead to more rapid processing of thyroid hormones and, in turn, affect TSH levels. • Treatment: synthroid or levothyroxine 13. Thyroid screening tests, confirmatory tests and monitoring There are differing opinions on whether to screen for thyroid disorders in asymptomatic adults. Adapted from the ATA/AACE Clinical Practice Guidelines for Hypothyroidism in Adults: Cosponsored by the American Association of Clinical Endocrinologists and the American Thyroid Association. Endocrine Practice, 18(6). Although there is no consensus about population screening in healthy individuals, there is evidence to support screening in the following at-risk populations, including those with • autoimmune disorders; • pernicious anemia; • first-degree relative with Auto-Immune thyroid disease; • history of neck radiation; • history of prior thyroid surgery or dysfunction; • abnormal thyroid examination; and • psychiatric disorders. Additional diagnoses that support thyroid screening are listed in Table 9 of the ATA/AACE Clinical Practice Guidelines. National organizations differ in their recommendations for routine screening in asymptomatic patients. In 2004 the U.S. Preventive Services Task Force (USPSTF) concluded that there was insufficient evidence for or against routine screening for thyroid disease in adults without symptoms, and the USPSTF has not updated this recommendation. If the patient is symptomatic or in a high-risk category, such as having a family history of thyroid disease or previous history of thyroid disease or autoimmune disorders, screening is appropriate. Confirmatory tests hyperthyroid: A 24-hour radioactive iodine uptake (RAIU) test can differentiate Graves’ disease from subacute thyroiditis and toxic nodular goiters, thereby refining treatment recommendations. DEQ – increased uptake It identifies areas of increased and decreased thyroid function, often termed hot and cold spots, within the gland. Patients with toxic nodular goiter and Graves’ disease have a high RAIU, whereas in subacute thyroiditis, iodine uptake is low. A thyroid scan is critical to determining functionality of any dominant thyroid nodule in a patient presenting with thyrotoxicosis, because cold nodules are highly suspicious for concomitant malignancy and must be evaluated further. An ultrasound of the thyroid will assist in differentiating a cyst from a nodule A fine-needle biopsy is the preferred initial diagnostic technique for evaluation of thyroid masses, particularly solid masses, to rule out malignancy. Magnetic resonance imaging is the preferred test to assess for ophthalmopathy resulting from Graves’ disease Monitoring hyperthyroidism: Before initiation of antithyroid therapy, a baseline complete blood count and liver function tests including hepatic aminotransferases (aspartate aminotransferase, alanine aminotransferase) should be obtained. During therapy, the white blood cell count is checked every 2 weeks during the first month and then every 4 to 6 months thereafter. Liver enzymes should be evaluated every 3 to 6 months. Radioactive Iodine Radioactive iodine–131 (131I; Iodotope) is the treatment of choice for hyperthyroidism in the United States, especially in middle-aged or older adults. Typically, a 24- hour radioiodine uptake dose of 75 to 200 mcCi per gram of estimated thyroid tissue is administered orally Women receiving radioactive iodine therapy should refrain from becoming pregnant for 4 months after therapy. T4 levels need to be checked monthly for 3 months after the administration of radioactive iodine in patients receiving radioactive thyroid ablation therapy. A nonradioactive alternative to thyroid radioablation for severe Graves’ disease or subacute thyroiditis involves administering a large quantity of concentrated iodine as a saturated solution of potassium iodide (SSKI, 35–50 mg iodide per drop, 1–2 gtts in water PO 2 times daily; Lugol solution, 8 mg iodide per drop, 3–5 gtts in water PO 3 times daily) or iopanoic acid (Telepaque, 1–3 g PO or 0.5 g PO 2 times daily). These concentrated iodine therapies effectively block the conversion of T4 to T3 and inhibit the release of thyroid hormones. Persons receiving radioactive iodine therapy should avoid contact with infants, children, and pregnant women for 7 days after ingestion. Women who receive this treatment postpartum must not breastfeed for at least 3 to 6 months, because radioactive iodine is excreted in breast milk and can ablate the infant’s thyroid. Screening and confirmatory tests hypothyroid: Thyroid Association recommends measuring thyroid function in all adults starting at age 35 years and then at least every 5 years. The American College of Physicians recommends TSH measurement in women older than 50 with one or more general symptoms that could be caused by thyroid disease. All patients with a prior history of any medically or surgically treated thyroid disease should be screened with a serum TSH measurement yearly. In addition, patients with other autoimmune diseases and those with unexplained depression, diabetes mellitus (DM), cognitive dysfunction, prior external radiation to the head and neck, hypercholesterolemia, or other risk factors should be screened with TSH measurements. Women experiencing unexplained infertility should be screened for thyroid dysfunction, and postpartum women with vague complaints may benefit from screening. Patients with secondary or tertiary (central) hypothyroidism show a low, normal, or mildly elevated TSH level with low FT4 and T3 by radioimmunoassay. The laboratory values for patients with subclinical hypothyroidism show a mildly increased TSH (4.5–10 mIU/L) with a normal FT4 level. The diagnosis of hypothyroidism is made by measuring serum TSH. TSH and FT4 should be used to follow treatment. When autoimmune thyroiditis is the suspected underlying cause, it is helpful to confirm this via antithyroid antibody titers, either antimicrosomal antibody (antithyroid peroxidase [TPO] antibody) or antithyroglobulin antibody. Once a diagnosis of hypothyroidism is confirmed, additional testing may be necessary to determine the effect of the disease on other body systems. Because T3 is nonspecific and not sensitive, it is not routinely used as an initial diagnostic tool. Because anemia is a frequent complication of hypothyroidism, a complete blood count should be done. A complete blood chemistry profile should be done to assess for alterations in electrolytes, blood urea nitrogen, creatinine, serum osmolarity, and glucose, because a decreased glomerular filtration rate (affecting renal function) can occur. A complete urinalysis should also be performed, with specific attention to the presence of protein (indicating possible renal impairment). Changes in the chemistries may be an indication of deteriorating thyroid function leading to myxedema. Monitoring: After therapy has been initiated with levothyroxine, the practitioner should check the patient’s levothyroxine levels in 4 to 8 weeks by evaluating the TSH level to determine whether adjustment of the levothyroxine dose is necessary. The target TSH level is 0.3 to 2.4 mIU/L. Increasing the levothyroxine dose more often than at 6- week intervals will probably lead to overreplacement. The patient should be examined annually for manifestations of thyrotoxicity (e.g., tachycardia, nervousness, or tremor) before increasing dosages. Laboratory values (FT4 and TSH levels) within normal limits and a satisfactory clinical exam suggest that treatment is adequate. For maintenance treatment, the medication should be titrated to the lowest dosage required to maintain euthyroidism, with a normal TSH and a normal or slightly elevated T4. Undetectable TSH levels suggest overtreatment; medication should be decreased in these patients. TSH levels greater than 10 mIU/L indicate undertreatment, and medication should be increased. Practitioners are encouraged to write prescriptions that do not allow substitution and use the same brand for the patient throughout treatment. The same brand of thyroid preparation is recommended because the bioavailability, stability, and content of the medication may vary with the different brands. 14. Risk factors for Hashimoto's & Grave’s disease Graves- gender(women), age (middle), autoimmune disorders(RA, DM 1, or lupus), stress, pregnancy, smoking, radiation exposure 15. Risk factors for secondary obesity • Risk factors for secondary obesity : short stature, slow growth velocity, delayed puberty, truncal obesity, and hirsutism • Disorders that cause secondary obesity: Cushing syndrome, PCOS, hypothyroidism, drug related • Secondary obesity is a medical condition that causes you to gain weight 16. Macrocytic vs microcytic vs normocytic anemia (causes, symptoms, testing) Mico- small size of RBCs, nutritional defocenience=iron. MCV value of less than 80 fL = Iron, anemia of chronic disease, thalassemias, sideroblastic (Prussian test). Lead poisoning test in children, rheumatoid arthritis. Macro- vitamin b12, folic acid, medications, pernicious anemia, liver damage. Normocytic- sepsis, hemorrhage, hemolysis, drug induced, aplastic, radiation, or hereditary spherocytosis. 17. X-linked disorders in African American males Sickle Cell (autosomal recessive), Sarcoidosis, AIDS, IBS, DM, HTN, Kidney disease, Alzheimer’s, Prostate cancer, panarctic cancer 18. Signs and symptoms of splenic sequestration Symptoms include sudden weakness, pale lips, fast breathing, extreme thirst, abdominal (belly) pain on the left side of body, and fast heartbeat. Inability to reuse iron. 19. Most accurate diagnostic testing for sickle cell anemia • Initial testing involves a CBC and a peripheral blood smear. They are typically diagnosed soon after birth and learn to cope with sickle cell crises even as they face delayed physical and psychological maturation. Postnatal infant screening in the United States, therefore, typically includes a hemoglobin electrophoresis to detect sickle cell disease and the various thalassemias. • Sickled cells will constitute 5% to 10% of the peripheral blood smear in most patients. The elevated reticulocyte count (>10% of the total erythrocyte count) is characteristically accompanied by the presence of Howell-Jolly bodies, which are small remnants of nuclear material from hemolyzed erythrocytes that reflect hyposplenia from autoinfarction, as well as target cells, which are erythrocytes with a deeply stained core surrounded by a lighter-stained margin that resemble a target with a bull’s eye. The clinician should also expect the WBC count to be elevated to 12,000 cells/mcL or greater, especially during and soon after a sickle cell crisis. Acute blood loss increased reticulocyte levels. • Further testing may include an indirect bilirubin level, which will be elevated after a sickle cell crisis due to hemolysis. Conversely, haptoglobin, a glycoprotein that binds free hemoglobin released from hemolyzed erythrocytes, will be significantly decreased, as it cannot be replaced quickly enough after a severe hemolytic episode. 20. Patient education for iron supplementation administration to improve efficacy Take on empty stomach. Iron tablets usually should not be taken with food, certain antibiotics, tea, coffee, calcium supplements, or milk. Iron should be taken one hour before or two hours after these items. If you take antacids, your iron tablets should be taken two hours before or four hours after the antacids. Iron tablets are best absorbed in an acidic environment; taking iron with one 250 mg vitamin C tablet or orange juice can enhance iron absorption. 21. Risks, diagnosis and treatment of hematological disorders 22. Common types of leukemia AML, ALL, CML, CLL ALL prevalent in pediatrics. CLL most common in type of Cx in United States. DEQ 23. Stages of chronic lymphocytic leukemia (CLL) Chronic lymphocytic leukemia (CLL) *CLL is the most common leukemia in adults. CLL starts in the cells that become certain WBC (lymphocytes) in the bone marrow as the cells change over to lymphocytes they are released into the blood. The cancerous T-lymphocytes increase SLOWLY and spread to other organs in the body such as lymph nodes, liver and spleen. This type of cancer may take a few years before any symptoms arise because it is very slow growing. *Lymphocytic leukemias (also known as lymphoid or lymphoblastic leukemia) start in the cells that become lymphocytes. DEQ. *Tumor size and spread aren't part of staging. Instead, blood and bone marrow tests plus spleen and lymph node size are used to stage this type of cancer. *The stage of a cancer is one of the most important things to know when deciding how to treat the cancer. Different types of CLL One kind of CLL grows very slowly and may take a long time before the patient needs treatment. The other kind of CLL grows faster and is a more serious disease. The leukemia cells from these 2 types look alike, but lab tests can tell the difference between them. The tests look for proteins called ZAP-70 and CD38. If the CLL cells have low amounts of these proteins, the leukemia tends to grow more slowly and have better long-term outcomes. Stages of chronic lymphocytic leukemia (CLL) Rai staging WBC subtype Organs Cells/platelet counts Risk Rai stage 0 Lymphocytosis (higher than normal) no enlargement of the lymph nodes, spleen, or liver red blood cell and platelet counts are near normal. low risk. Rai Stage I Lymphocytosis (higher than normal) enlarged lymph nodes; spleen and liver are not enlarged red blood cell and platelet counts are near normal. intermediate risk Rai stage II Lymphocytosis (higher than normal) enlarged spleen (and maybe an enlarged liver) lymph nodes may or may not be enlarged; red blood cell and platelet counts are near normal. intermediate risk Rai stage III Lymphocytosis (higher than normal) lymph nodes, spleen, or liver may or may not be enlarged red blood cell counts are low (anemia); platelet counts are near normal. high risk Rai stage IV Lymphocytosis (higher than normal) enlarged lymph nodes, spleen, or liver red blood cell counts may be low or near normal; platelet counts are low (thrombocytopenia). high risk Binet staging system In the Binet staging system, CLL is classified by the number of affected lymphoid tissue groups (neck lymph nodes, groin lymph nodes, underarm lymph nodes, spleen, and liver) and by whether or not the patient has anemia (too few red blood cells) or thrombocytopenia (too few blood platelets). If CLL is suspected the testing will include ❖ CBC—Will show a WBC elevation ❖ Peripheral blood smear-Will show lymphocytes that occupy as much as 90% of the smear. ❖ Platelet count—elevated and rarely diminished ❖ Bone marrow biopsy—will confirm the initial peripheral blood smear as well, as small, mature lymphocytes dominate the field. Monoclonal surface immunoglobulin on the malignant lymphocytes aids in distinguishing these small cells form the normal counterparts. ❖ Secondary test—immunoelectrophoretic will further support the diagnosis of CLL in about ½ of patient, as the profile from the electrophoresis demonstrates hypogammaglobulinemia. 24. Acute lymphoblastic leukemia (ALL) treatment in children and correlation with subsequent cancers in adulthood The main treatment with ALL is chemotherapy. Given in three phases induction, consolidation, and maintenance. Typical treatment is 2-3 years, most intense in the first few months. Induction phase: Goal of induction is remission. Remission means leukemia cells are no longer found in bone marrow samples. 95% of children with ALL enter remission after 1 month of induction treatment. First month is intense and prolonged and requires frequent hospital stays, due to the serious infections or complications that may occur. Children with standard-risk ALL often receive 3 drugs for the first month of treatment. Chemotherapy L-asparaginase and vincristine, and a steroid drug such as dexamethasone. High risk children receive a fourth chemo medication anthracycline class, most often daunorubicin. Other drugs that may be considered include methotrexate and/or 6-mercaptopurine. Children with a Philadelphia chromosome-positive ALL also benefit from targeted therapy, such as the drug imatinib (Gleevec). All children receive chemo through CSF to kill leukemia cells that have spread to the brain or spinal cord. This is intrathecal chemotherapy and is given through a lumbar puncture. Usually given twice or more if the child is high risk or leukemia cells have been found in the CSF, during the first 1-2 months. Some may be given radiation therapy to the brain, however, this is avoided most often. Possible side effect of intrathecal chemo is seizures. Consolidation phase: More intense, starts once the leukemia is in remission and typically lasts several months. Further reduces the number of leukemia cells in the body. Standard risk treatment: Treatment with methotrexate, 6-mercaptopurine (6-MP), vincristine, L-asparaginase, and/or prednisone. High risk treatment: L-asparaginase, doxorubicin (Adriamycin), etoposide, cyclophosphamide, and cytarabine (ara-C). Dexamethasone may be used instead of prednisone. A second round of chemo may be used, this is known as delayed intensification. Philadelphia chromosome-positive ALL: additional target therapy such as imatinib (Gleevec) Stem cell transplant may be needed for high risk children, once the leukemia is in remission Maintenance phase: If leukemia remains in remission, maintenance therapy can be started. Most plans use 6-MP and weekly methotrexate given as pills. Along with vincristine which is IV and a steroid either prednisone or dexamethasone. These drugs are given for brief periods every 4-8 weeks. Some children at high risk receive more intense maintenance chemo or intrathecal therapy. If the leukemia comes back during treatment, then the child will receive more intense chemotherapy. Stem cell transplant is often recommended for children whose leukemia does not go into remission and an appropriate donor match must be found. There is correlation with childhood cancers to adulthood cancers, and this increases if the childhood cancer occurred before age 15. The increased risk is related to a hereditary cancer syndrome, treatments that were performed that may have caused cancer-causing side effects, or due to risk related to age. Family history can also play a role. Brain tumors may result. - DEQ 25. Animal bites • Epidemiology: Most often animal bites are from a dog and most often in children under 12. Infectious organisms: E. Coli, pasteurella, corynebacteruim and alpha-hemolytic strep. Cat bite organism P. multocida results in intense inflammation and joint infection. • Clinical presentation: Infection evidenced by increased pain, swelling, erythema, warmth, decreased range of motion. Be aware of systemic signs such as regional lymphadenopathy, fever etc when the patient does not present for days after the bite. • Diagnostics: xray indicated if fracture is suspected, cultures from wound if patient immunocompromised, if patient is septic, or when antibiotics have failed. Labs are only indicated if patient is seriously ill. • Management: • Pain control with OTC's or Torsdol if severe pain. • Animal bites are highly infectious! Clean with mild soap and water, 1% Betadine. If rabies if suspected irrigte with 1% benzalkonium chloride. Punctures should be debrided to 1-2mm rim for drainage. Bite wounds on hands should not be sutured, usually only facial wounds that present immediately after the bite get sutured. If after 3-5 days no purulence or erythema wound closure may be performed. • Bites are tetanus prone. Tetanus vaccine booster if has immunization series but no booster within 5 years. If unsure or not immunized within ten years 250 units of tetanus immune globulin should be given with the vaccine. • Patient education: **If the dog is a pet that has been vaccinated, rabies is not suspected, so clean with soap and water and treat like any other wound** 26. The treatment and management of wounds Four phases of healing: and lacerations 1) Injury phase, 2) Inflammatory phase, 3) Epithelialization phase, and 4) Remodeling phase. Also referred to as: 1. Homeostasis phase=Inflammatory phase 2. Proliferation or migratory phase=Epithelialization phase 3. Maturation and remodeling phase=Remodeling phase Infection with Pasteurella multocida characteristically develops rapidly following cat or dog bites with erythema, swelling, and intense pain evident as early as 12 to 24 hours after the bite. Aerobic and anaerobic blood cultures are warranted prior to antibiotic therapy in patients with an infected bite wound and signs of systemic infection. Amoxicillin/clavulanate twice daily for 3 to 5 days is the agent of choice. Cefuroxine is an alternative with less reported GI side effects. For patients with penicillin or cephalosporin allergies, prophylaxis with an agent with activity against Pasteurella such as Doxycycline or Bactrim DS is recommended 27. Assessment and administration of tetanus vaccine for post exposure management • Bite wounds are tetanus-prone injuries. If patient has not received a tetanus booster within the last 5 years, a booster should be administered. For patients with an absent or incomplete primary immunization, 250 units of tetanus immune globulin should be given. 28. Erectile dysfunction ED may result from a combination of both physical and psychological or emotional causes and treatments (psychogenic) causes because such etiologies are not mutually exclusive. Several categories of ED should be considered when determining the cause of the problem. A failure to generate the nerve impulse required to initiate an erection may be caused by a number of endocrinological or neurologic conditions, or it can be psychogenic in origin. ED can also be the result of a failure to adequately fill penile blood vessels, most often arteriogenic in origin. Another category is the failure to retain blood in the penis, which is usually veno- occlusive in origin. Nonpsychogenic Causes of Erectile Dysfunction Neurologic Diseases • Anterior temporal lobe lesions • Disease of the spinal cord • Loss of sensory input (secondary to diabetes mellitus, polyneuropathies), tabes dorsales (disease of dorsal root ganglia) • Disease of nervi erigentes (secondary to complete prostatectomy, retrosigmoid operations, aortic bypass) Vascular Disease • Leriche syndrome Endocrine Disorders • Testicular failure (primary or secondary) • Hyperprolactinemia Penile Disorders • Failure of detumescence • Priapism • Penile trauma • Peyronie’s disease Medications • Phenothiazines • Thioridazine • Imipramine • Methyldopa • Guanethidine • Reserpine • Spironolactone • Alcohol • Heroin • Methadone • Estrogen • Beta blockers • Thiazide diuretics • Antihypertensives A number of options are available for the treatment of ED. If an organic cause cannot be found, these men will most likely benefit from behaviorally based sex therapy. Pharmacologic treatments including hormone therapy. Nonpharmacologic interventions including vacuum constriction devices, vasoactive therapy, penile prostheses, and penile revascularization are discussed in the sections that follow. Hormone Therapy For men with documented testosterone deficiency who do not have prostate cancer or benign prostatic hypertrophy (BPH), breast cancer, or cardiovascular disease, testosterone therapy is the treatment of choice. However, testosterone therapy should not be used in men with high blood pressure, clotting disorders, and may increase the severity of sleep apnea. Testosterone therapy can be accomplished by several delivery methods that include injections, oral medication, topical patches, or topical gels. Vacuum Constriction Devices Most vacuum devices work in similar ways, using a process that takes about 2 minutes. The patient inserts his penis into the cylinder, then uses the pump to create a partial vacuum. This causes venous blood to enter the corpora cavernosa, initiating tumescence and rigidity. Once a sufficient erection is achieved, a latex constriction ring is placed around the base of the penis to help maintain the erection. Vasoactive Therapy The development of drugs that decrease the breakdown of 5-cyclic guanosine monophosphate (cGMP) has revolutionized ED treatment. cGMP is the intracellular second messenger of nitric oxide, which is the primary vasodilator and neurotransmitter involved in the erectile response. The first of these drugs was sildenafil citrate (Viagra). Penile Prostheses Several prosthetic devices that can be surgically implanted into the penis are available in a variety of sizes and diameters. They are placed directly in the corporal bodies. Penile prostheses may be rigid, semifirm, hinged, or inflatable. Penile Revascularization The experience with penile revascularization is limited, and some patients fail to have a sufficient erection even after the procedure. Patients with arterial disorders may be candidates for the various procedures, which include endarterectomy and balloon dilation, or arterial bypass. For patients with venous disorders, ligation of the deep dorsal vein or emissary vein or ligation of the crura of the corpora cavernosus may be effective. Low-Intensity Shock Wave Therapy Low-intensity shock wave therapy (LIST) has met with success in Europe in patients with severe ED who are unresponsive to treatment with PDE5 inhibitors such as sildenafil (Viagra), tadalafil (Cialis), and vardenafil (Levitra). It is not yet approved in the United States, but research is ongoing. It is believed that LIST promotes the release of angiogenic factors that lead to revascularization of the penis. 29. Drug of choice for treatment of priapism The diagnosis of priapism is made based on visual inspection of the penis, which reveals an erection that has been present for more than two to four hours in the absence of sexual excitation. Treatment of ischemic priapism of less than four hours’ duration is a intracavernosal injection of a sympathomimetic drug (eg, phenylephrine). After four hours’ duration, aspiration combined with an intracavernosal injection of a sympathomimetic drug is considered to be the optimal treatment. Phenylephrine, a pure alpha-adrenergic agonist, is considered the sympathomimetic of choice for ischemic priapism Once ischemic priapism has been ruled out, observation alone is appropriate for initial management of nonischemic priapism If patients do not respond to repeated cavernous aspiration and alpha-agonist therapy, shunt surgery is the next treatment option. 30. Hypospadias a birth defect (congenital condition) in which the opening of the urethra is on the underside of the penis instead of at the tip. The urethra is the tube through which urine drains from your bladder and exits your body. Surgery usually restores the normal appearance of child's penis. With successful treatment of hypospadias, most males can have normal urination and reproduction. Opening of the urethra at a location other than the tip of the penis symptoms- Downward curve of the penis (chordee), Hooded appearance of the penis because only the top half of the penis is covered by foreskin, Abnormal spraying during urination 31. Treatment of undescended testes An undescended testicle is usually corrected with surgery. The surgeon carefully manipulates the testicle into the scrotum and stitches it into place (orchiopexy). This procedure can be done either with a laparoscope or with open surgery. Hormone treatment involves the injection of human chorionic gonadotropin (HCG). This hormone could cause the testicle to move the scrotum. Hormone treatment is not usually recommended because it is much less effective than surgery. saline testicular prostheses. Should descend by age of 2; otherwise surgery is needed before age 6 for normal maturation and hormone function – DEQ. 32. Varicocele vs testicular torsion vs hydrocele vs epididymitis Testicular torsion: Most common symptom is testicular pain followed by swelling. Typically, sudden onset and often spontaneous occurrence (only associated with traumatic events in 20% of cases). Torsion results in loss of fertility from the affected teste in 90% of cases. Absence of the cremasteric reflux is commonly noted. Elevating the testes does not relieve pain (which helps to distinguish testicular torsion from epididymis). Most often diagnosed based on physical examination alone but can be confirmed with Doppler ultrasound. Irreversible necrosis can happen in as little as 6 hrs or less. Surgery to salvage the affected testicle is most successful when performed within 4 hrs. Pt education: As many as 67% of salvaged testes will atrophy within 2-3 years even after successful detorsion surgery. Testicular torsion is a twisting or rotation of the testes around the spermatic cord, which is the blood supply to the testes. The lack of blood supply to the testes results in acute ischemia. This condition is considered a urological emergency, so in the primary care setting, you must be able to recognize this and immediately refer the patient to the ER. Compression of the testicular vessels will lead to ischemic necrosis within 6 hours, so failure to recognize the torsion and intervene immediately can result in loss of the testicle. Testicular torsion can happen at any age, even to newborns and older men; however, most cases are seen in adolescent and young adult males. It is not a common condition and its etiology is not clear, but one theory is that contraction of the cremasteric muscle may contribute. Things that can cause contraction of the muscle include trauma, exercise (most frequent in runners), extreme cold, and sexual stimulation. The Differential Diagnosis for torsion should include • epididymitis; • acute varicocele; • acute hydrocele; • incarcerated hernia; and • traumatic hematoma.• History and Physical Exam The most common symptom in testicular torsion is sudden, severe pain accompanied by swelling of the affected testis. The patient may have pain for several days without seeking medical attention. The most common finding on clinical exam is the absence of the cremasteric reflex and unlike in epididymitis elevation of the affected testis does not relieve the pain (Phren’s sign). This finding is not enough to differentiate between the two conditions, but rather they can support your suspicion Varicocele: VW (bag of worms) Varicoceles are caused by weak walls and vascular engorgement in the spermatic cord. You can think of it as varicose veins of the testes. Abnormal degree of venous dilation (varicosity) of the pampiniform plexus in the spermatic cord above the testes, which may result in pain and engorgement of the testis. It is most often described as a soft mass above the testicle which feels like a “bag of worms.” Varicocele commonly results in decreased male fertility—present in as many as 50% of men with decreased fertility. Grade 1 varicocele is palpable only when the patient performs the Valsalva maneuver. A Grade 2 varicocele is palpable when the patient is standing. A Grade 3 varicocele may be assessed with light palpation and visual inspection. Referral to urologist is indicated. Surgical intervention through venous ligation often improves fertility/sperm quality. f fertility is not a concern, treatment is conservative with NSAIDs and scrotal support. Varicocele can be bilateral, but if it is unilateral it is almost always on the left side due to the anatomy of the vasculature drainage in the testes. On physical exam with the patient sitting upright, tortuous veins located posterior and above the testes can be seen. Venous engorgement may increase with the Valsalva maneuver. When the patient lies down in the recumbent position, the distension will resolve Hydrocele-peritoneal fluid w/in the scrotum around the testes Causes-acute epididymitis, tuberculous epididymitis, trauma to the testes, tumor of the testes, or sequelae as complication of radiation therapy, exstrophy of bladder, pt w/Ehlers-Danlos have increased risk for hydrocele as do pt w ventricular peritoneal shunt for dialysis Hydroceles in infants result from failure of the patent processus vaginalis to close. Hydrocele of spermatic cord forms when distal processus vaginalis closes but midportion remains patent and filled w/fluid. Rapidly forming hydroceles result from reactive inflammatory processes w/I the scrotum Subjective: swelling in scrotum or inguinal canal, usually painless but may have heaviness, if pain is present it may radiate to low back Objective: when the scrotum is transilluminated w a penlight the trapped fluid appears lt pink, yellow, or red Diag test: US, testicular nuclear scan is used to distinguish testicular torsion, abdominal xray can distinguish an incarcerated hernia from a hydrocele Causes of hydrocele include • acute epididymitis; • teste trauma; • testicular tumors; and • sequelae of radiation therapy complications. History findings will complain of swelling in the scrotum or inguinal canal. They are usually painless and are reported as a sense of heaviness in the scrotum. The physical exam should include an examination of the scrotum, testes, abdomen, and groin. Additionally, transillumination of the scrotum should be performed. To do this, in a darkened room, place a penlight up against the scrotum. He trapped fluid from a hydrocele will appear light pink, yellow, or red. The testes themselves do not illuminate, and neither does a hematoma. This is typically considered a diagnostic finding. Differential Diagnosis • indirect inguinal hernia; • orchitis (infection of testes); • epididymitis; and • varicocele. • Rule out hernia – hernia does not transilluminate DEQ Epididymitis-inflammation of the epididymis results in scrotal pain, swelling, and induration of the posterior-lying epididymis, eventually may result in scrotal wall edema and involve the adjacent testicle-may also be known as epididymo-orchitis Predisposition-unprotected intercourse, h/o UTI w/dysuria, or urethral discharge Symptoms may occur after heavy lifting Most common in sexually active men or older men with UTI Causes in males <35-STD like chlamydia or gonorrhoeae Causes in males >35-coliform bacteris (E Coli) or sometimes Pseudomonas auruginosa or Staph aureus, can be associated with distal urinary tract obstruction, tuberculous epididymitis presents with sterile pyuria, another cause is sterile urinary reflux following transurethral prostatectomy or from bacilli Calmette-Guerin intravesical therapy for superficial bladder cancer Subjective:scrotal pain that radiates along spermatic cord or to flank, may be acute over several hours, may have pain at the tip of penis and complain of urethral discharge, dysuria, cloudy urine, or hematuria. Elevation will relieve discomfort. Fever and chills w/severe infection or abscess Objective: scrotal swelling, testis may be indistinguishable from epididymis. Nodularity of the vas deferens and tenderness of the epididymis, non STD epididymitis will have pyuria. Rectal exam may reveal a tender prostate. Diag test: urinalysis-pyuria and leukocytosis, STD testing may show gonorrhoeae or chlamydia. CBC shows increased WBC with left shift to more immature forms Testicular Cancer Compared with other types of cancer, testicular cancer is rare and is highly treatable. Testicular cancer is the most common cancer in American males between the ages of 15 and 35. There is no clear cause and effect relationship for testicular cancer, but risk factors include • HIV infection; • Caucasian race; and • history of cryptorchidism (undescended testes). Other risk factors that have been identified include • higher social status; • being unmarried; and • living in a rural area. Differential diagnosis of testicular cancer should include • epididymitis; • hydrocele; • varicocele; • hematoma; and • spermatocele. 33. Signs and symptoms of chronic vs acute prostatitis Acute bacterial prostatitis – always associated with UTI and abrupt • General complaints: fever, chills, low back pain, malaise, arthralgia, myalgia • Urinary complaints: frequency, urgency, dysuria, nocturia, bladder-outlet obstruction • Physical examination: warm, tense, boggy, very tender prostate Chronic bacterial prostatitis – **most common** • General complaints: symptoms often absent, perineal pain, low back pain, lower abdominal pain, scrotal pain, penile pain, pain on ejaculation • Urinary complaints: dysuria, irritative voiding • Physical examination: normal, boggy, or focally indurated prostate History of recurrent UTI Nonbacterial prostatitis – similar to chronic but no evidence of infection • General complaints: pelvic pain • Urinary complaints: irritative voiding, abnormal flow • Physical examination: similar to chronic bacterial prostatitis, tender on palpation Prostatodynia – s/s of prostatitis; no inflammation Common bacteria – E. coli and staph aerus Obstructive symptoms: - what is obstructing? Prostate? What is it affecting? Weak stream Incomplete bladder emptying Dribbling Irritative symptoms: - if you have this, why what would be irritating? Urinary complaints – increased urgency, hesitancy, DRE results: Do not press hard can cause sepsis 34. Management and intervention for BPH Benign Prostatic Hyperplasia/Benign Prostatic Hypertrophy(BPH) • BPH common name for nodular hyperplasia of prostate • affects men older than 40 y/o • prostate gland enlargement There are 2 mechanisms of obstruction in BPH: static constriction and dynamic constriction. In static constriction, direct obstruction of the bladder neck is caused by a build-up of prostatic tissue. In dynamic constriction, constriction of the bladder neck is caused by an increase in prostatic muscle tone through adrenergic stimulation. Obstruction of the bladder outlet forces the bladder to generate higher pressures than normal to achieve micturition. Increased muscle mass in the bladder leads to reduced bladder elasticity and compliance resulting in a reduction in bladder capacity. Diagnostic testing • urinalysis and urine C&S are done to exclude infection and hematuria • PSA is done to assess for prostate cancer • urine cytology is done to exclude bladder cancer • the AUA no longer recommends a serum creatinine on the initial evaluation should be done on subsequent visits or if obstructive uropathy is present. More advanced diagnostics include, should be done by a urologist: • urodynamic studies to identify difficulties in emptying the bladder • post-void residual urine test (PVR) • cystoscopy Alpha1-adrenergic antagonists, which relaxes the smooth muscle around the neck of the bladder or 5-alpha-reductase inhibitors, which block the conversion of testosterone to DHT. Medical Management: - can be treated as outpatient- goal is to relieve symptoms of dysfunctional urination- nocturia - inpatient treatment – required to manage fluid and electrolyte imbalance - avoid caffeine, alcohol, highly seasoned foods- bladder irritants Common A1A medications prescribed include: Smooth muscle relaxers • Tamsulosin (Flomax) 0.4mg-0.8mg QD • Doxazosin (Cardura) 4-8mg QD • Silodosin (Rapaflo) 4-8mg QD 5- alpha-reductase inhibitors that are commonly prescribed are: • Finasteride (Proscar) 5mg QD alone or in combination with Doxazosin • Dutasteride (Avodart) 0.5mg QD Recap: - Selective alpha1 adrenergic antagonist- need to be titrated • Silodosin (Rafaflo) 4-8 mg daily o Taken with meal o C/I severe renal/hepatic impairment • Trazosin ( Hytrin) 1-10 mg daily • Doxazocin (Cardura) 1-8 mg daily - Alpha 1A adrenergic blockers- no titration needed/taken immediately after same meal each day • Tamsulosin (Flomax) 0.4 or 0.8 mg daily • Alfuzosin (UroXatral) 10 – 15 mg daily - 5 alpha reductase inhibitors- decrease PSA concentration by 50% • Finasteride (Proscar) • Dutasteride ( Avodart) - Saw palmetto • Available in tablet, liquid, or tea form: 320 mg by mouth daily. • Inhibits 5-α-reductase activity and mimics the effects of finasteride; combination of saw palmetto, lycopene, and selenium is thought to be more effective • PSA levels may be artificially lowered by saw palmetto - African wild potato (Hypoxis hemerocallidea) • Sixty to 130 mg of β-sitosterol (a constituent of African wild potato) divided into two or three doses daily. It acts by decreasing inflammation. Invasive and Surgical management - Transurethral resection of the prostate- (TURP) o Surgical tx of choice o performed through a cystoscope, using a diathermy loop for the resection of prostatic tissue o Flow-stream rates are increased as much as 15 mL/s o Complications=retrograde ejaculation and erectile dysfunction - transurethral incision of the prostate (TUIP) o an instrument is passed through the urethra to make one or two cuts in the prostate and the prostatic capsule, reducing the urethral stricture in order to relieve symptoms o can be done outpatient basis - Open prostatectomy o surgical option reserved for prostate glands weighing more than 40 to 100 g. o surgical removal of the inner portion of the prostate via a suprapubic or retropubic incision in the abdominal area. - Robotic simple prostatectomy o used for patients with very large prostates. o inner part of the prostate that is causing the obstruction is removed - transurethral laser–induced prostatectomy (TULIP) o is a type of coagulation necrosis and is done under transrectal ultrasound guidance. - transurethral electrovaporization of the prostate o grooved roller-blade electrode administers diathermic energy to the prostate, vaporizing the prostatic tissue. o It provides improved urinary flow through the urethra - Transurethral microwave thermotherapy (TUMT) o microwave energy is applied to the prostate through a microwave antenna in a urethral catheter; temperature monitoring of the rectum and urethra is essential - Transurethral needle ablation (TUNA) o two radiofrequency energy waves are administered to prostatic tissue via needle electrodes applied through a cystoscope. o Temperatures up to 120°F (48.9°C) destroy the prostatic tissue that had caused the urinary retention or obstruction - high-intensity focused ultrasound o rectal probe delivers a short burst of high-intensity focused ultrasound energy that heats the prostate tissue, resulting in coagulative necrosis. - Transurethral (balloon) dilation of the prostate o involves the insertion of a catheter with a balloon at the end through the urethra and into the prostatic urethra - Selective prostatic artery embolization (PAE) o alternative treatment for those with prostates greater than 80 mL in size who failed medical treatment and are unsuited for surgery. 35. Assessment and management of prostate Prostate CA Assessment: cancer SUBJECTIVE: Usually asymptomatic early in the disease process and may even be asymptomatic in late stages of disease. • bone pain, weight loss, anemia, shortness of breath, lymphedema, and lymphadenopathy • Neurologic symptoms (e.g., an inability to perceive touch, pain, and temperature in the perineal or scrotal areas and a lack of sensation of bladder distention) occur with epidural metastasis and cord compression. • Patient complaints can also include bladder-outlet symptoms or acute urinary retention with very large or locally extensive tumors, although these symptoms are most often due to BPH. OBJECTIVE: Prostate exam reveals palpable hard prostate. Nodules or induration may be noted as well. Late signs: hematuria, hemosperia Evaluation of contraction of the rectal sphincter in response to the bulbospongiosus (bulbocavernosus, Osinski) reflex in which the glans penis is squeezed should direct attention to a possible lesion at the level of the conus medullaris of the spinal cord. DIAGNOSTICS: PSA (prostate specific, NOT cancer specific) Screening: (American Cancer Society) • 50 years of age for men with little to no risk factors • 45 years of age for black men and men who have a father, brother, or son diagnose with prostate cancer before age 65. • 40 years of age for men at higher risks PSA levels may be elevated in individuals with BPH, but with regard to cancer screening, traditionally a PSA level greater than 4.0 ng/mL is considered positive and a level of 4.1 ng/mL has been considered a threshold for performing prostatic biopsy. ***DRE, TRUS, and Biopsy to diagnose STAGING: Prostate tumors are classified according to the Gleason system in which an initial grade (score) is applied to the architectural pattern of the cancer in the largest segment of the specimen and then a second grade is given to the next largest area. The pathologist adds the two scores together to produce the Gleason score, which is on a scale from 1 to 10. Accurate staging provides an indication of the best treatment options. • Gleason score 1 to 4: indicates a well-differentiated cancer that is likely to be slow growing • Gleason score 5 to 7: indicates a moderately differentiated cancer • Gleason score 8 to 10: indicates a poorly differentiated cancer that is likely to be aggressive and rapidly growing TREATMENT: Depends on age. If the cancer has not spread; surgery, radiation therapy, watchful waiting and/or chemotherapy. If the cancer has spread, it is typically fatal and only palliative methods are performed. MEDS: Hormone therapy if warranted (estrogen, LH antagonists) [Show More]
Last updated: 1 year ago
Preview 1 out of 46 pages

Reviews( 0 )
Document information
Connected school, study & course
About the document
Uploaded On
Jun 26, 2022
Number of pages
46
Written in
Additional information
This document has been written for:
Uploaded
Jun 26, 2022
Downloads
0
Views
35