Chemistry > Lab Report > Laboratory 5 – Titration of a Strong Acid CHE 201 – General Chemistry I Spring semester – 2020 (All)
Laboratory 5 – Titration of a Strong Acid CHE 201 – General Chemistry I Spring semester – 2020/ 2021 - Section 002 | Download for quality grades |
Document Content and Description Below
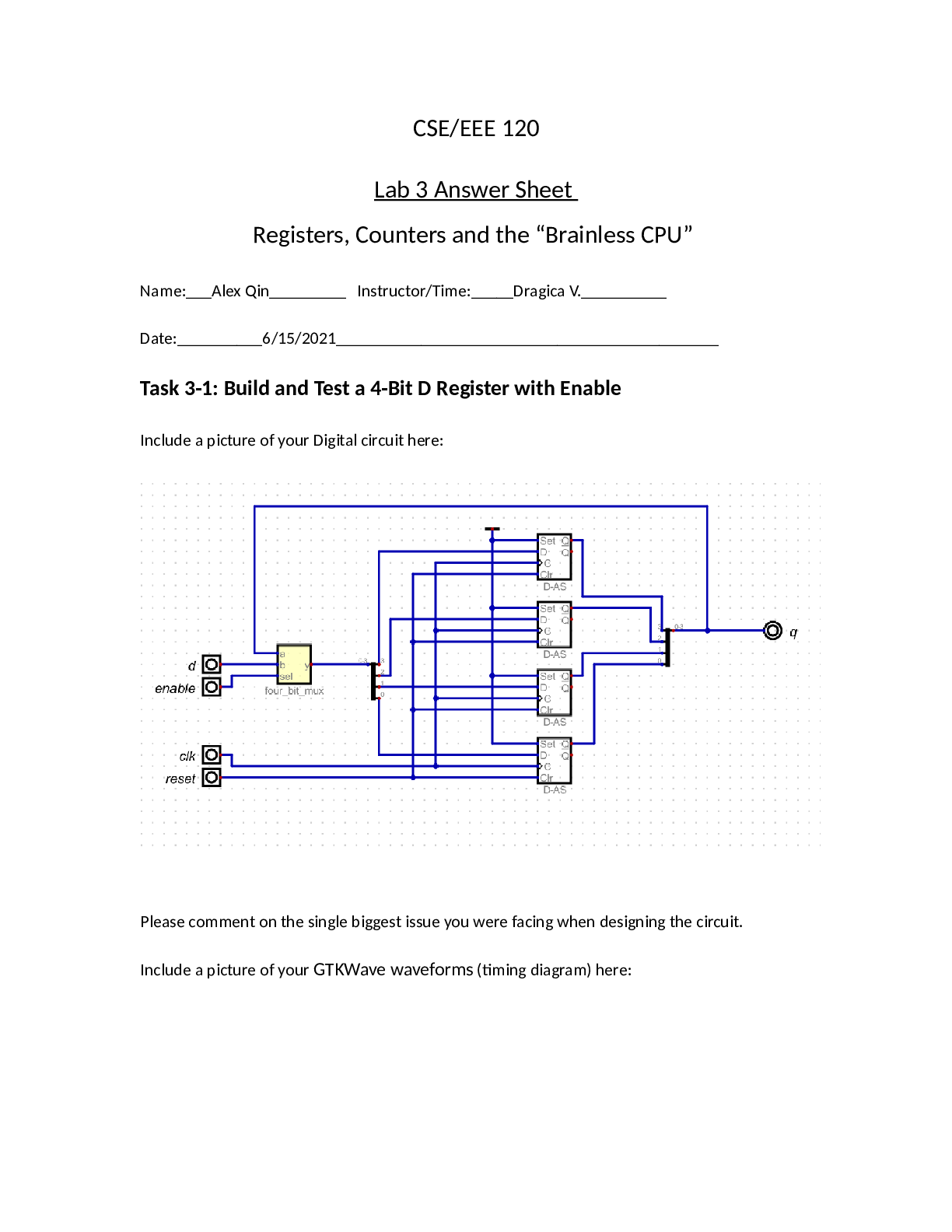
Titration of a Strong Acid Page 2 Abstract (done by Fatima) In this experiment, we use a process of chemical analysis called Titration. The aim of using this process is because it is useful when y... ou are giving a solution you don’t know the molarity event, by performing titration you will get to know concentration of any solution. We start with filling the burette with NaOH solution, and in a flask we put 25 ml of HCL solution and 4 drops of phenolphthalein indicator, we start adding the NaOH to the HCL and the indicator then we swirl it gently, while we are adding the NaOH we realized that the color is shown gradually until it reached the light pink, we repeat the experiment twice to make sure our experiment was successful and it was! because the volume of NaOH was 0.01328 mol/dm3 and the volume of HCL was 0.0282 mol/dm3. Introduction (done by Mariam W) Titration of a strong acid with a strong base is the simplest of the four types of titrations as it involves a strong acid and strong base that completely dissociate in water, thereby resulting in a strong acid strong base neutralization reaction. This titration requires the use of a buret to dispense a strong base into a container of strong acid, or vice-versa, in order to determine the equivalence point (Chemistry LibreTexts, 2019). The purpose of a strong acid-strong base titration is to determine the concentration of the acidic solution by titrating it with a basic solution of known concentration, or vice-versa, until neutralization occurs. As both the acid and base are strong (high values of Ka and Kb), they will both fully dissociate, which means all the molecules of acid or base will completely separate into ions (Khan Academy, 2019). At the equivalence point, equal amounts of H+ and OH- ions will combine to form H2O, resulting in a pH of 7.0 (neutral) [Show More]
Last updated: 1 year ago
Preview 1 out of 6 pages

Reviews( 0 )
Document information
Connected school, study & course
About the document
Uploaded On
Mar 26, 2023
Number of pages
6
Written in
Additional information
This document has been written for:
Uploaded
Mar 26, 2023
Downloads
0
Views
80














 (1).png)