Chemistry > DISCUSSION POST > CHEM120 Week 5 Virtual Lab: Organic Chemistry – Download For An A+ (All)
CHEM120 Week 5 Virtual Lab: Organic Chemistry – Download For An A+
Document Content and Description Below
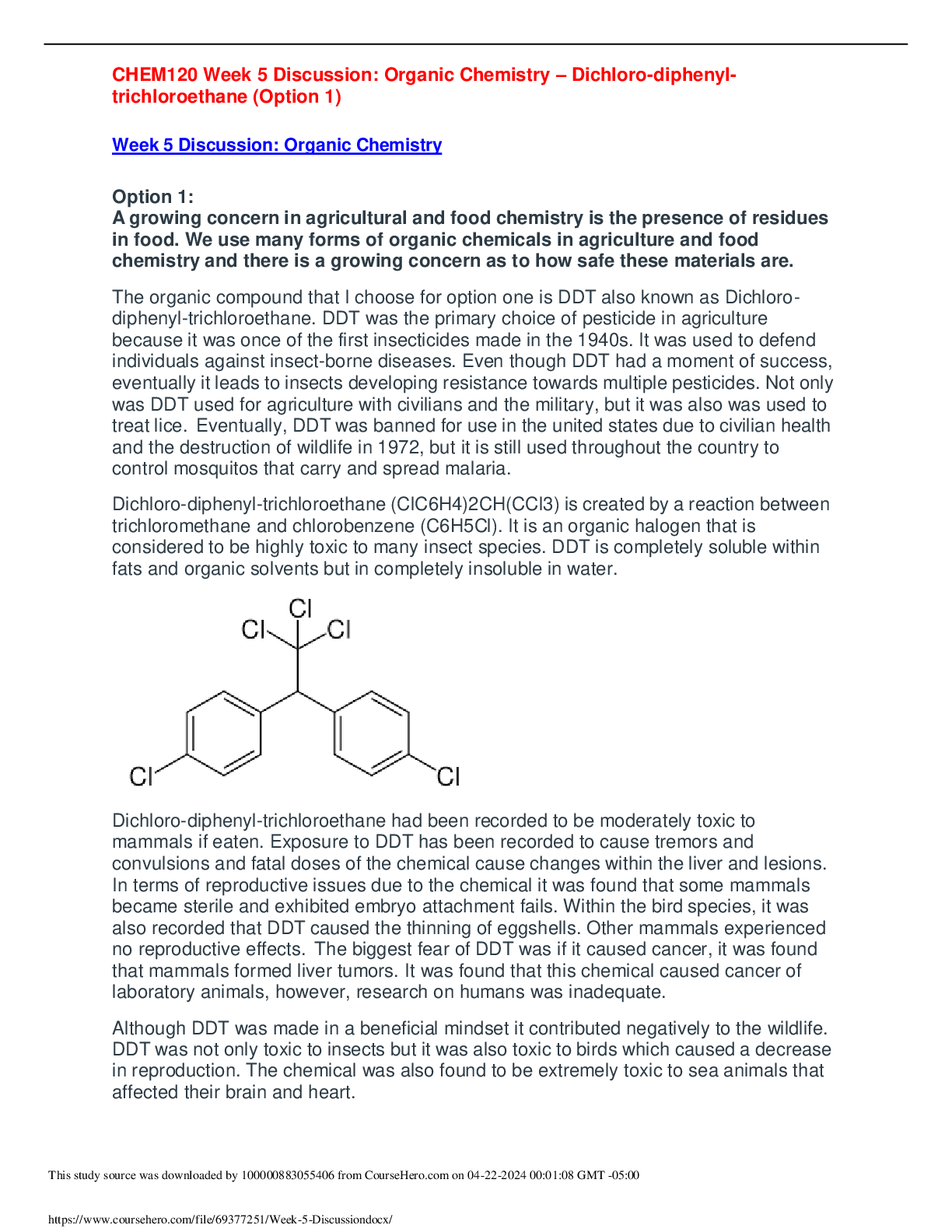
CHEM120 Week 5 Virtual Lab: Organic Chemistry – Download For An A+ OL Lab 9: Building models of organic compounds Learning Objectives: • Build virtual models to learn about the structure o... f organic compounds • Draw extended structural formulas of organic compounds Compounds that are based on the carbon atom are known as organic compounds. These compounds commonly contain, nitrogen, oxygen, and hydrogen in addition to carbon. Carbon forms a variety of covalent compounds with varied properties. Carbon containing compounds are formed by sharing electrons, covalent bonds, between atoms. Most biomolecules, as well as most drugs, are classified as organic compounds. In this laboratory exercise, you will build models of organic compounds virtually and draw the extended structural formula of organic compounds. Online Modeling Resource: http://molview.org/ Note: Be sure to build each of the compounds as instructed using the online modeling resource kit. This hands-on experience is an important part of this lab. You will need to copy the models you build in the virtual modeling resource and paste the images into this document. Please take the time to explore the structures of these organic compounds. Exploration 1: Building models of Hydrocarbons Hydrocarbons are a diverse group of organic compounds containing carbon and hydrogen. Hydrocarbons can be linear, branched, or cyclic. Additionally, hydrocarbons can be saturated, unsaturated or aromatic. Using the virtual resource build the extended structural formulas of the following compounds. Copy and paste the images into the space below. Additionally, type in the condensed structural formula. Propane Virtual Model with Extended Structural Formula: Butane Virtual Model with Extended Structural Formula: Condensed Structural Formula: CH3CH2CH3 Condensed Structural Formula: CH3CH2CH2CH3 Isobutane Virtual Model with Extended Structural Formula: Condensed Structural Formula: (CH3)2CHCH3 Isopentane Virtual Model with Extended Structural Formula: Condensed Structural Formula: (CH3)2CHCH2CH3 Ethylene Ethyne Virtual Model with Extended Structural Formula: Condensed Structural Formula: C2H4 Virtual Model with Extended Structural Formula: Condensed Structural Formula: C2H2 Cyclohexene Benzene Virtual Model with Extended Structural Formula: Virtual Model with Extended Structural Formula: Condensed Structural Formula: C6H10 Condensed Structural Formula: C6H6 Propyne Extended Structural Formula: Ethane Virtual Model with Extended Structural Formula: Condensed Structural Formula: CH3CCH Condensed Structural Formula: CH3CH3 Exploration 2: Identification of Functional Groups Part 2A: Building functional groups Functional groups alter the properties of hydrocarbons. Using the virtual resource build the extended structural formulas of the following compounds. Copy and paste the images into the space below. Alcohol Ether Ketone Carboxylic acid Aldehyde Ester Amine Part 2B: Identification of functional groups: Complete the table below by identifying the functional groups and names of the condensed structural formulas below. Condensed Structural Formula Name of Functional Group Name of Organic Molecule CH3CH2COCH3 Ketone 2-Butanone CH3CH2CHO Aldehyde Propanel CH3OH Alcohol Methanol CH3CH2CH2CH2CH2NH2 Amino Aminopentane CH3CH2CH2COOH Carboxylic Acid Butanoic Acid Exploration 3: Building hydrocarbons containing functional groups Using the virtual resource build the extended structural formulas of the following compounds. Copy and paste the images into the space below. Difluoromethane Virtual Model with Extended Structural Formula: Trichloromethane Virtual Model with Extended Structural Formula: Propanol Virtual Model with Extended Structural Formula: Ethanoic Acid Virtual Model with Extended Structural Formula: Combine the propanol and the ethanoic acid from the last two exercises to make propyl ethanoate Virtual Model with Extended Structural Formula: Condensed Structural Formula: C2H6O Propanal Virtual Model with Extended Structural Formula: Hexanoic acid Virtual Model with Extended Structural Formula: Condensed Structural Formula: CH3CH2CH2OH Condensed Structural Formula: C6H12O2 Ethylamine Virtual Model with Extended Structural Formula: Questions: 1. Write the names of a biomolecule (also known as macromolecules) that contain each of the functional groups below. a. Amine – Amino Acids b. Aldehyde - Carbohydrates c. Carboxylic Acid – Amino Acids d. Alcohol – Glucose/ Carbohydrates and sugar, Amino Acids 2. Find an example of an ester used as a fragrance or flavoring and give the name, condensed structural formula, and flavor of your chosen ester. Pentyl butonate-Apricot C3H7COOC5H11 3. For each of the following, give the functional group and application a. Formaldehyde Functional Group- Aldehyde Application- Anti-bacterial and acts as a preservative b. Ethanol Functional Group- Alcohol Application- Main ingredient in alcohol, like beer. c. Acetone Functional Group- Carbonyl Application- Nail polish remover and manufacturing like plastic d. Phenol Functional Group- Hydroxyl Application – Used in drugs and household cleaners Reflection: Consider what you learned from this simulation. Reflect on three to four key concepts that you learned in this lab exercise. How could the lessons learned in this virtual lab relate to a real-world situation in the community/world or your future career? Be specific in your answer (this should require 5-10 sentences). • Carbon containing compounds are formed by sharing electrons, and covalent bonds. • Hydrocarbons can be saturated, unsaturated, or aromatic. • Most biomolecules are classified as organic compounds. In this weeks’ lab I learned that functional groups like Hydroxyl, Alcohol and Aldehydes play an important role in things like household cleaners, the nail polishes we use and most importantly the alcohol we consume. This has given me a new perspective on how much chemicals are in things we use daily without realizing it . Grading Rubric: Activity Deliverable Points Exploration 1: Build/draw all hydrocarbons: 5 points 5 Exploration 2: Build/draw all functional groups: 3.5 points and complete the table: 5 points. 8.5 Exploration 3: Build/draw all organic molecules: 5.5 points, 5.5 End of lab questions Complete all questions: 9 points total (Questions 1 and 3 are 4 points each, question 2 is 1 point) 9 Reflection Write a 10-14 sentence laboratory reflection.: 7 point s 7 All Lab Deliverables Complete ALL explorations and reflection activities 35 [Show More]
Last updated: 2 weeks ago
Preview 1 out of 20 pages

Reviews( 0 )
Document information
Connected school, study & course
About the document
Uploaded On
Apr 22, 2024
Number of pages
20
Written in
Additional information
This document has been written for:
Uploaded
Apr 22, 2024
Downloads
0
Views
7



























Growth and developmental patterns of toddlers.png)



