Hunter College, CUNY - CHM 376Exam #1_2012
Document Content and Description Below
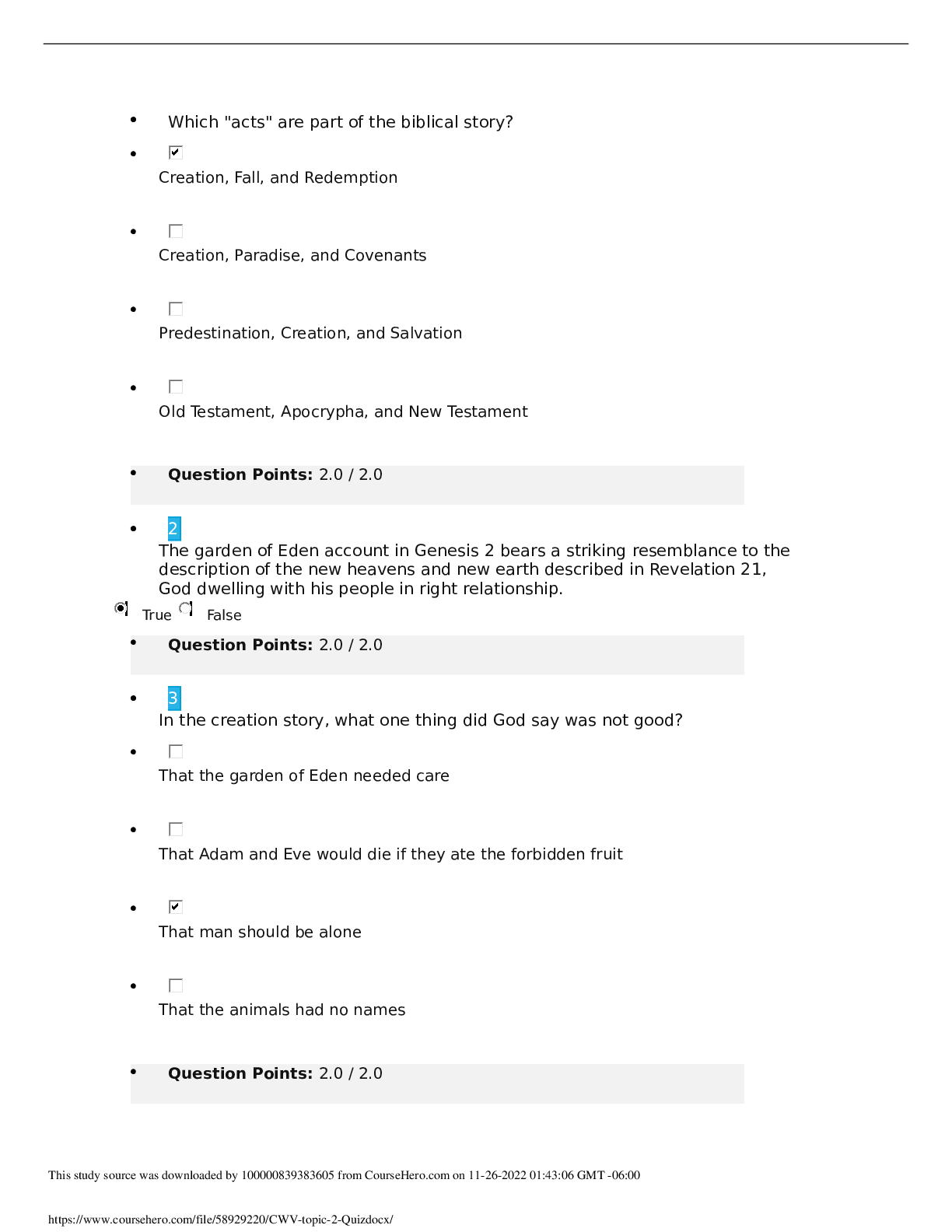
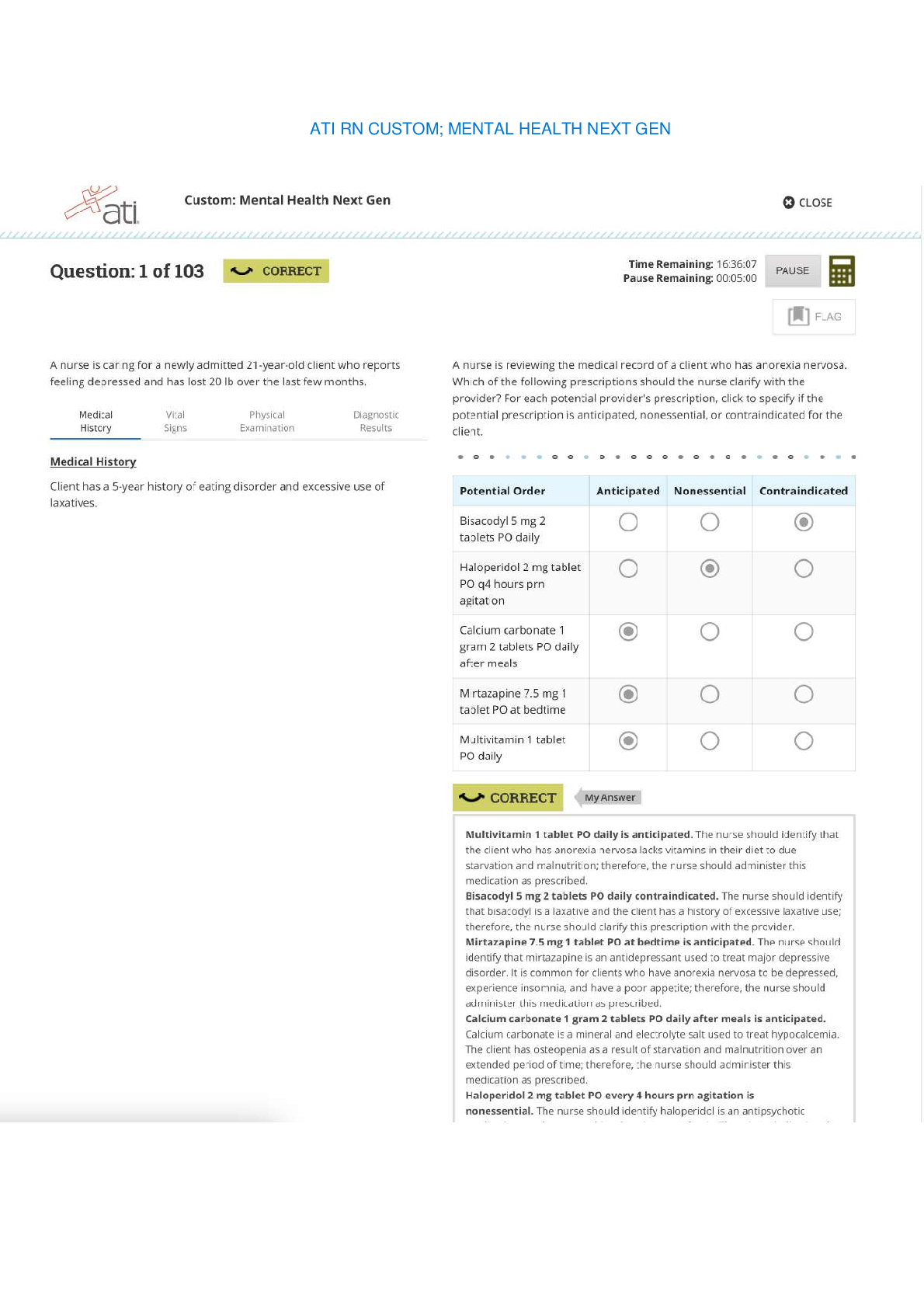
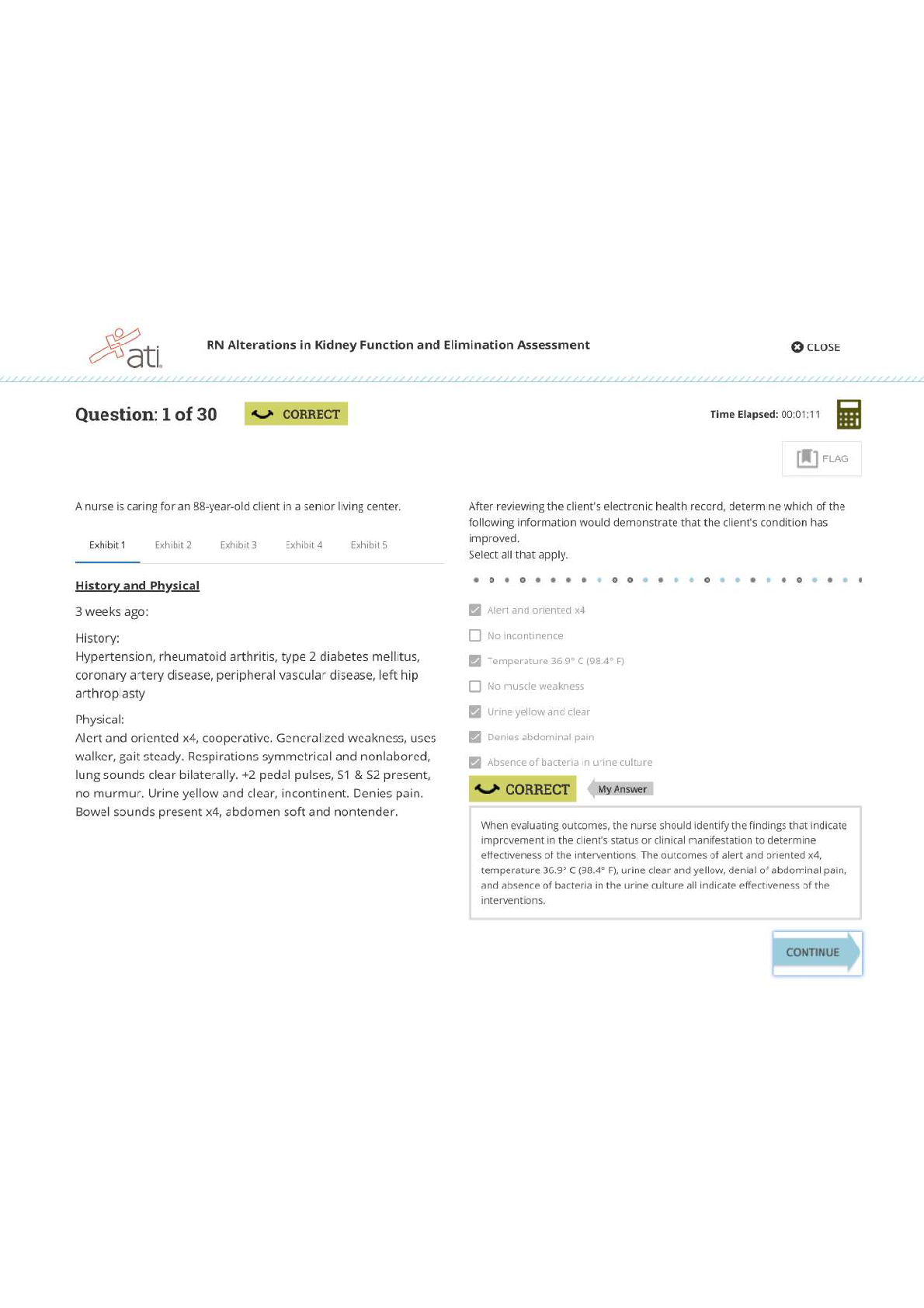
CHM 376/640 Fall 2012 October 8, 2012 Exam #1 You are bound by the Academic Honor Code. This means: 1) you will not give or receive information during this exam, nor will you consult unauthorized... sources of information; 2) you will not discuss the contents of this exam with other students until all students have finished the exam; and 3) you will not tolerate violations of academic integrity on the part of other students – if you see someone else cheating, please bring it to the attention of a proctor immediately. Please sign: ___________________________________ Time allowed for this exam: 4:10 – 5:25 p.m. Note: 1) For all calculations, it is only necessary to use ONE significant digit beyond a decimal point in any of the values and your answer; to simplify calculations for this exam only, this includes approximating values of constants, etc., to one digit beyond the decimal point; 2) A number of the questions have more than one part – please be sure to answer each part! 3) You have two detachable sheets at the back of the exam, one with a basic log table and values for constants, and one blank sheet to be used for calculations or for scratching out answers because WE WILL ONLY GRADE NEATLY WRITTEN ANSWERS WITHIN THE SPACES PROVIDED; 4) Time is limited, so I recommend that you look over the entire exam (and point values of questions) first, and plan your time accordingly; 5) Show ALL work so that you can receive partial credit for correct calculations even if a final answer is wrong; 6) If the pages of your exam come apart, please put your last name on each page (upper right) and I will staple them together after you hand it in; 7) If something is not clear to you, raise your hand and someone will answer your question; 8) THERE ARE SEVEN PROBLEMS WITH A TOTAL OF 107 POINTS (but exam will be graded as your score out of 100 pts).Page 1 ______________________ 1. (19 points total) Answer the following questions briefly but clearly and specifically. Please note that questions include several parts, and that the question continues on the next page). a) (5 pts) The phosphate group transfer potential (i.e. the Ghydrolysis) of ATP is considerably greater (G° = 30.5 kJ/mol) than that of hydrolysis of the phosphoester bond of glucose6 phosphate (G° = 10 kJ/mol)? Please explain why by providing an overall reason (in terms of components of G°) and 3 different supporting properties that contribute to the overall G°. 1) overall reason: 2) specific: 3) specific: 4) specific: b. (6 pts) How does a dipoledipole interaction compare with a hydrophobic interaction? Please answer in terms of (1) the origin of each effect; and (2) the electrostatic properties of the particles involved and the nature of the interaction between them [Show More]
Last updated: 1 year ago
Preview 1 out of 11 pages
.png)
Reviews( 0 )
Document information
Connected school, study & course
About the document
Uploaded On
Mar 31, 2021
Number of pages
11
Written in
Additional information
This document has been written for:
Uploaded
Mar 31, 2021
Downloads
0
Views
28






.png)
.png)
.png)
.png)
.png)
.png)
.png)
.png)