Chemistry > Lab Report > CHEM 120: Unit 4 Lab 7- Ideal Gas Law (All)
CHEM 120: Unit 4 Lab 7- Ideal Gas Law
Document Content and Description Below
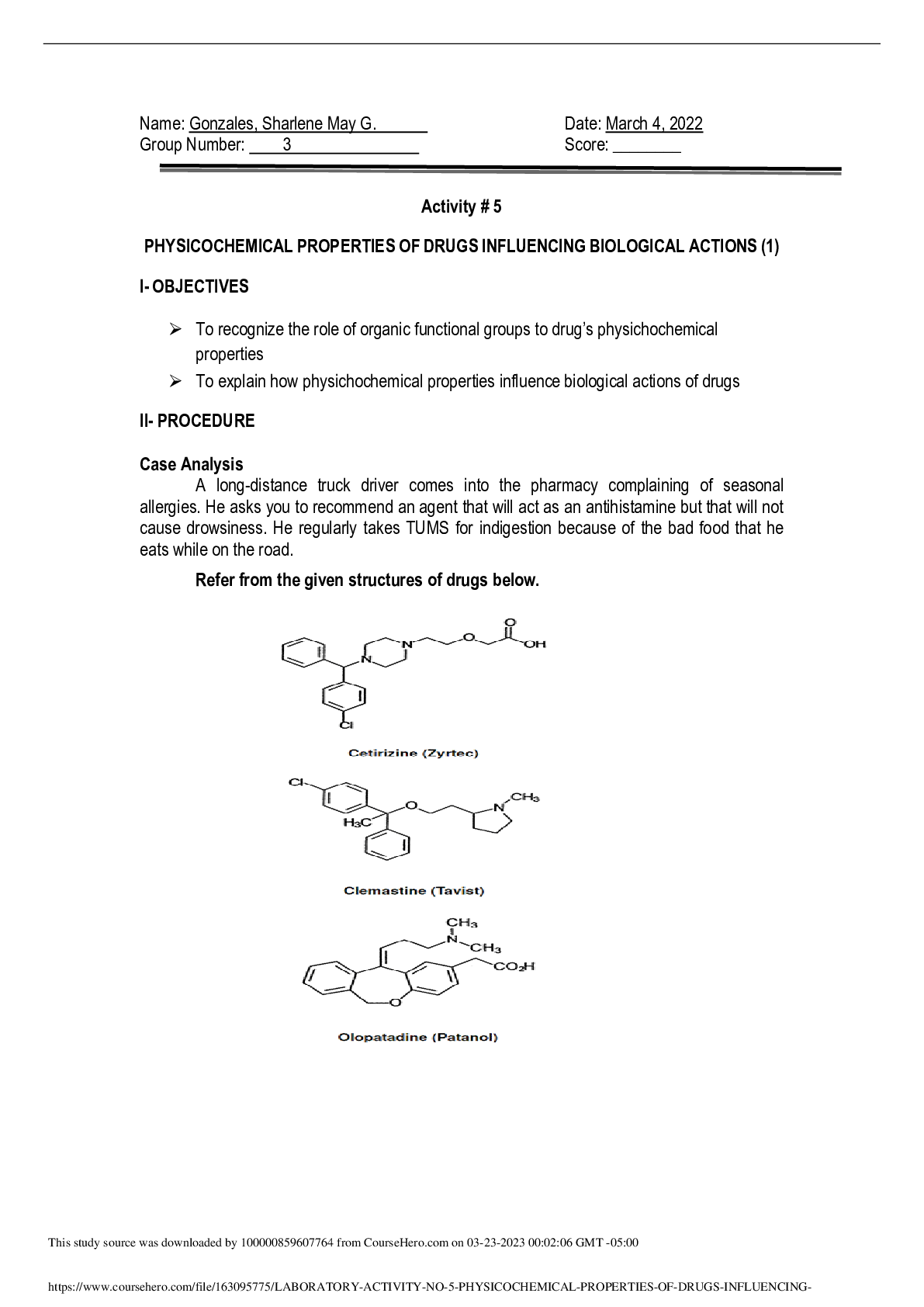
Part 2: Report and Reflection Purpose: Describe in complete sentences and in your own words, the purpose of this experiment. The purpose of this experiment was to define the physical concept of tem... perature and absolute zero. Also, to observe how ideal gas molecules behave according to the ideal gas law. The last thing will be to understand the relationship between pressure, volume and temperature in gases using thermometry. Observations: Record three observations from the simulation. 1. The temperature scale is an arbitrary scale. 2. The burner will increase the temperature of the glass jar. The high temperature will increase the average kinetic energy. 3.increasesd temperature and consequently increased pressure is the outcome of gas molecules having more kinetic energy. Answer the questions below: 1. If the pressure of a fixed volume of gas decreased in a sealed container, what variable would you think changed? Did this variable increase or decrease? The variable that changed is the pressure. When the volume decreases the pressure increases. 2. Why is it important to convert into units of Kelvin before using the Ideal gas law? It is important to convert into units of Kelvins before using the ideal gas law because the pressure and volume of gas depends on the kinetic energy or motion of particles. 3. Using what you learned in this simulation, explain why compressed gas cylinders, such as those found in the hospital, typically contain a warning to not leave in sunlight or expose to heat. These gas cylinders contain warnings to not leave in sunlight or expose to heat because they can explode, create a fire since they react to changes. 4. Reflection: Consider what you learned from this simulation. Reflect on three to four key concepts that you learned in this lab exercise. How could the lessons learned in this virtual lab related to a real world situation in the community/world or your future career? Be specific in your answer (this should require 5-10 sentences). What I learned from this virtual simulation is that absolute zero is constant and regardless of minor variations due to experimental errors we should always be considered as equal to -273.15. I also learned that the initial pressure in the dipper should be double and that the volume occupied by the molecules themselves is no longer negligible. This can help me I n my future career as a nurse to understand that transplants need to be in a certain temperature as well as how to use the oxygen tanks. [Show More]
Last updated: 1 year ago
Preview 1 out of 4 pages

Reviews( 0 )
Document information
Connected school, study & course
About the document
Uploaded On
Aug 02, 2021
Number of pages
4
Written in
Additional information
This document has been written for:
Uploaded
Aug 02, 2021
Downloads
0
Views
47













 (1).png)




.png)
.png)


