Chemistry > Lab Report > Lab Report > CHE MISCEquilibrium Lab Report 07.06. (All)
Lab Report > CHE MISCEquilibrium Lab Report 07.06.
Document Content and Description Below
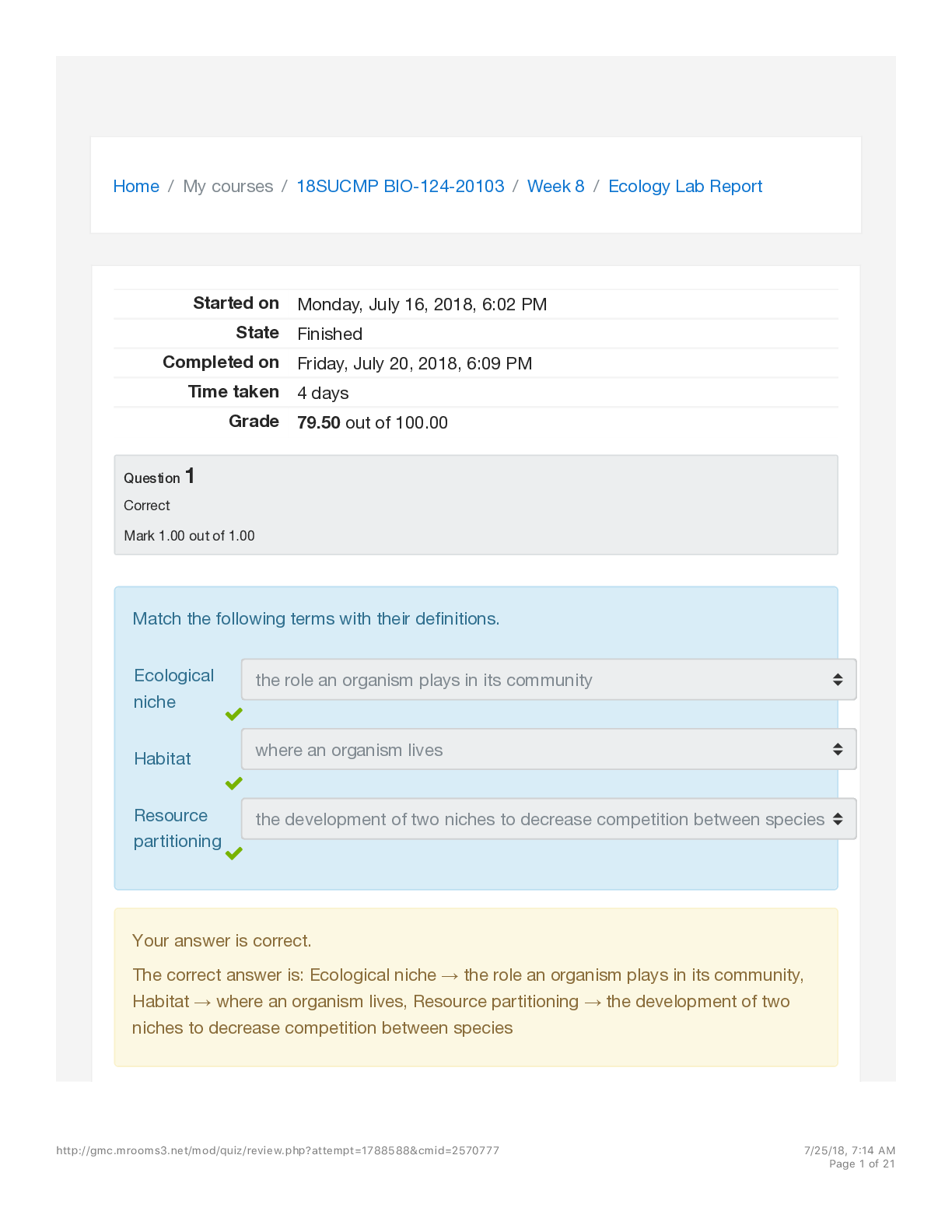
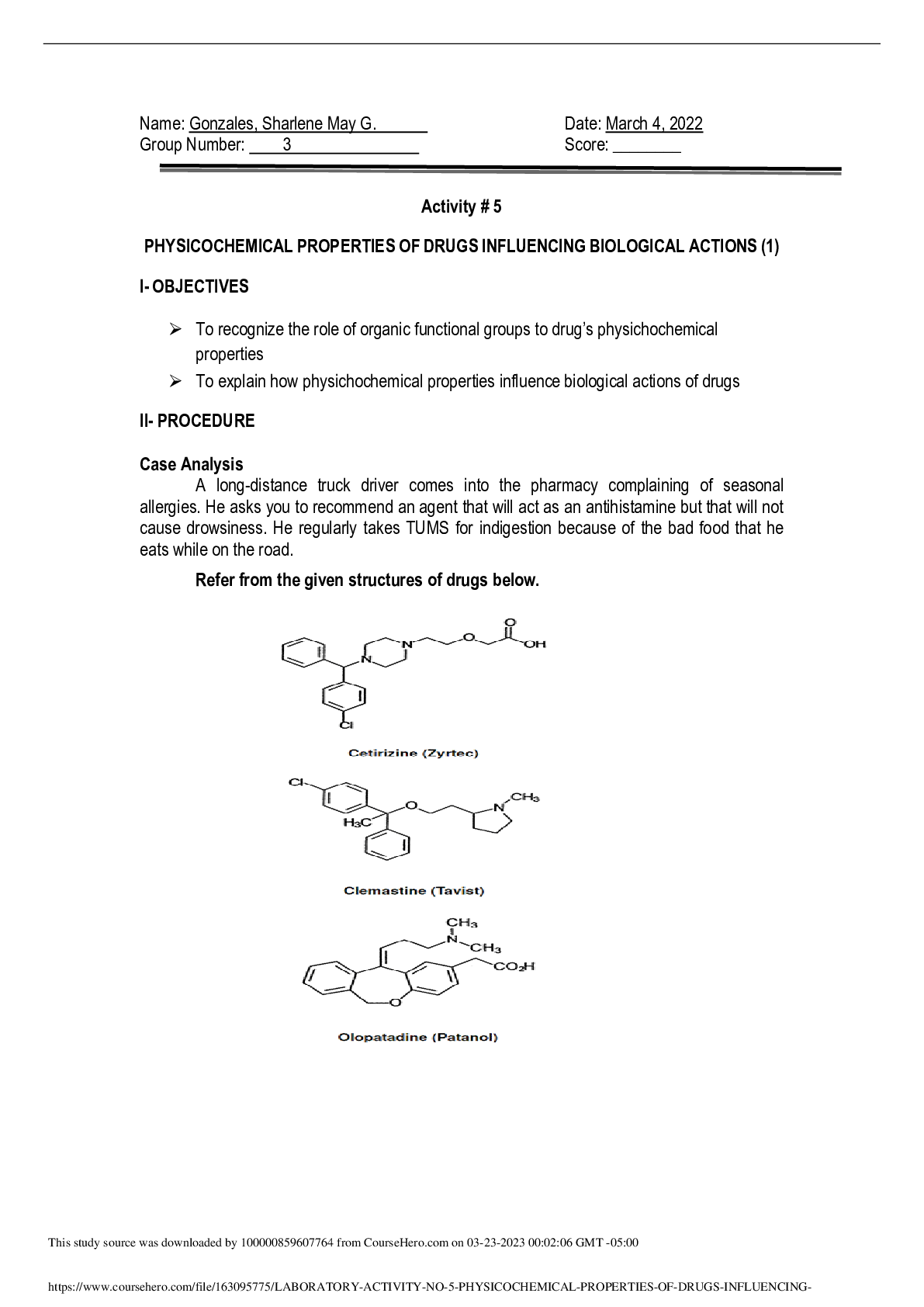
Equilibrium Lab Report Instructions: For this investigative phenomenon, you will observe the changes that result when you add more of, or take away some of, one of the components of the equilibrium (... known as a stress) and determine if there are trends in the behavior of equilibrium systems. Record your observation in the lab report below. You will submit your completed report. Title: Equilibrium Lab Objective(s): To study what factors affect equilibrium of reactions. Hypothesis: If the amount of reactant increases, the rate of the reverse reaction will increase to reach a new equilibrium. Explain the relationship between forward and reverse reactions at equilibrium and predict how changing the amount of a reactant or product (creating a stress) will affect that relationship. For example (select one from each underlined section) If the amount of (reactant or product) increases, the rate of the (forward or reverse) reaction will (increase or decrease) to reach a new equilibrium. If the amount of (reactant or product) decreases, the rate of the (forward or reverse) reaction will (increase or decrease) to reach a new equilibrium. Explain the relationship between forward and reverse reactions at equilibrium and predict how changing the amount of a reactant or product (creating a stress) will affect that relationship. For example (select one from each underlined section) If the amount of (reactant or product) increases, the rate of the (forward or reverse) reaction will (increase or decrease) to reach a new equilibrium. If the amount of (reactant or product) decreases, the rate of the (forward or reverse) reaction will (increase or decrease) to reach a new equilibrium. Procedure: Access the virtual lab and complete the inquiry experiment. List your controlled variables, independent variable, and dependent variable. Explain why these are the variables. Summarize the steps of the experimental procedure. Materials: Equilibrium Virtual Lab Variables: Remember, controlled variables are factors that remain the same throughout the experiment. An independent (test) variable changes so that the experimenter can see the effect on other variables. The dependent (outcome) variable will change in response to the test variable. Controlled variables: amount of chemicals added and amount of solutions Independent variable: what is added to solutions Dependent variable: color of solutions Summary of Steps: Include a summary of steps here. We have included the first two important steps to get you started. You can summarize the rest from the virtual lab. 1. Select each control test tube to compare the concentrations of the reactants and products. Then record this data 2. Get you 4 test tubes of Co(H2O) 6 2+ and CoCl4 2- at approximately equal concentrations. 3. Add a solution of HCI to test tube 1, stir with clean, dry stirring rod. Record the color change and graph data. 4. Take test tube 1 and add a solution of AgNO3 . Stir with a clean, dry stirring rod. Record the color change and graph data. 5. Add liquid H2O to test tube 2. Stir with a clean, dry stirring rod. Record the color change and graph data. 6. Add NaCl salt to test tube 3. Stir with a clean, dry stirring rod. Record the color change and graph data. 7. Add liquid H2O to test tube 3. Stir with a clean, dry stirring rod. Record the color change and graph data 8. Add solid CaCl2 salt to test tube 2. Stir with a clean, dry stirring rod. Record the color change and graph data. 1. Write down the equilibrium equation you are investigating using the information provided by the introduction within the virtual lab activity Equilibrium reaction: Co(H2O) 6 2+ + 4Cl ⇋ CoCl4 2- + 6H2O 2. Select each control test tube to compare the concentrations of reactants and products. Write down what each indicator color means about the concentrations of reactants and products in solution. Pink means: When there is significantly more Co(H2O) 6 2+ than CoCl4 2- in the test tube, the solution is pink, as in the control test tube A. Blue means: When there is significantly more CoCl4 2- then Co(H2O) 6 2+ in the test tube, the solution is blue, as in the control test tube B. Purple means: When the amounts of Co(H2O) 4 2+ and CoCl4 2- are approximately equal in the test tube, the solution is purple,as in control test tube B Data: For each trial, make sure you record the initial color of the solution, what is added to the test tube (a description of the stress), and what happened after the substance was added. Also include a screen shot, sketch, or description of each graph of each trial. ::::::::::::::::::::::::::::::::::::::::CONTENT CONTINUED IN THE ATTACHMENT::::::::::::::::::::::::::::::::::::::::::::::::::: [Show More]
Last updated: 1 year ago
Preview 1 out of 12 pages

Reviews( 0 )
Document information
Connected school, study & course
About the document
Uploaded On
Feb 10, 2021
Number of pages
12
Written in
Additional information
This document has been written for:
Uploaded
Feb 10, 2021
Downloads
0
Views
264


 (1).png)















 (1).png)


.png)