Chemistry > Lab Experiment > Lab6 - CUNY Hunter College CHEM 225.LB (All)
Lab6 - CUNY Hunter College CHEM 225.LB
Document Content and Description Below
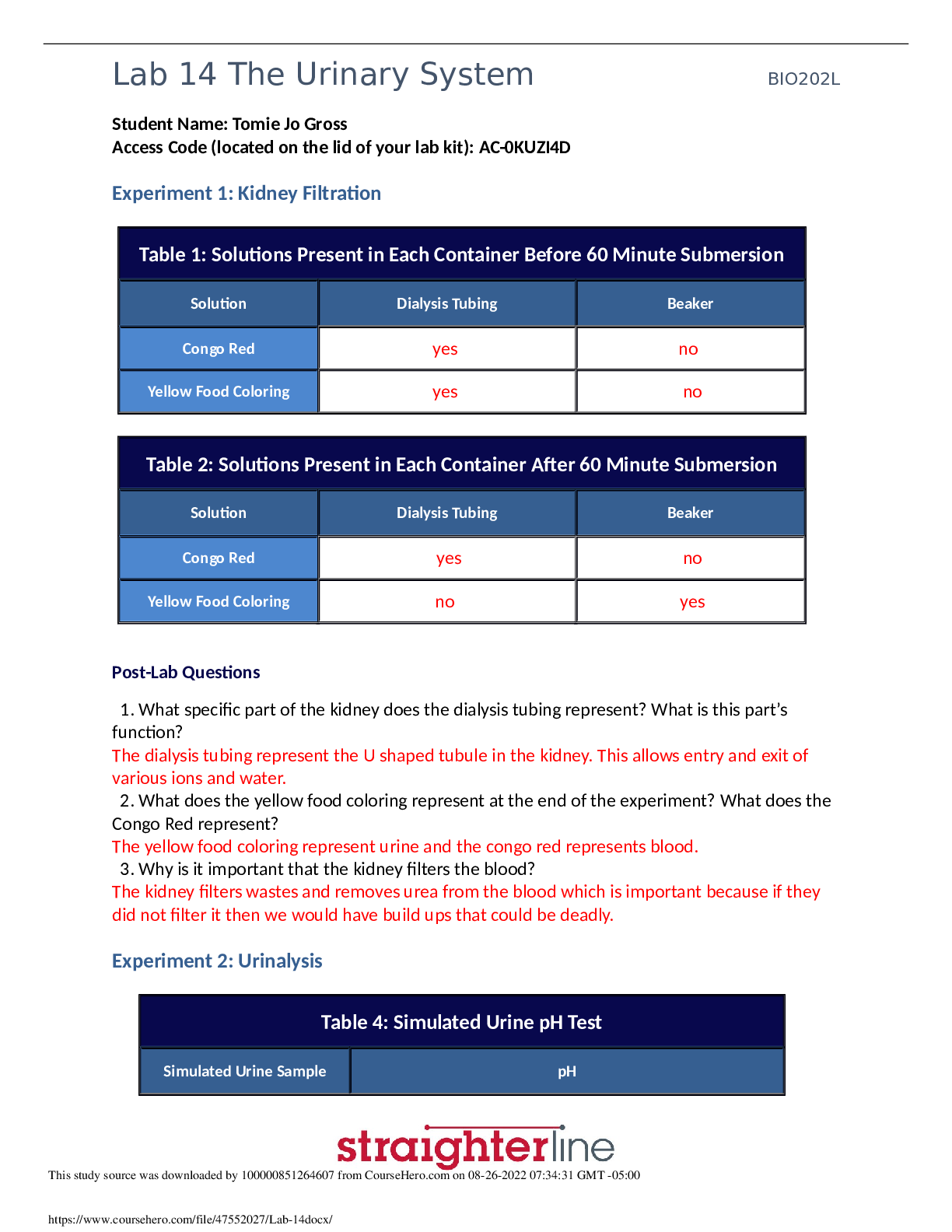
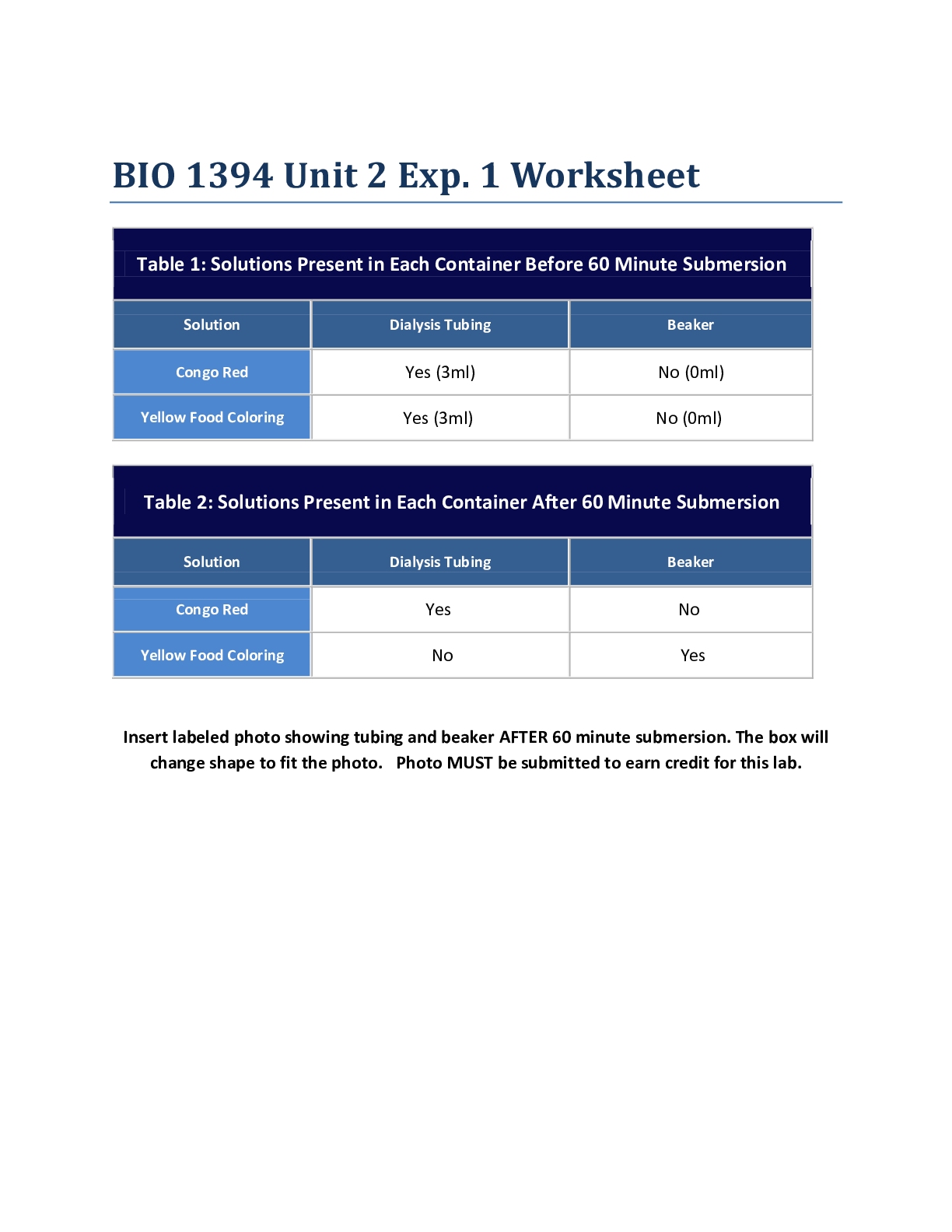
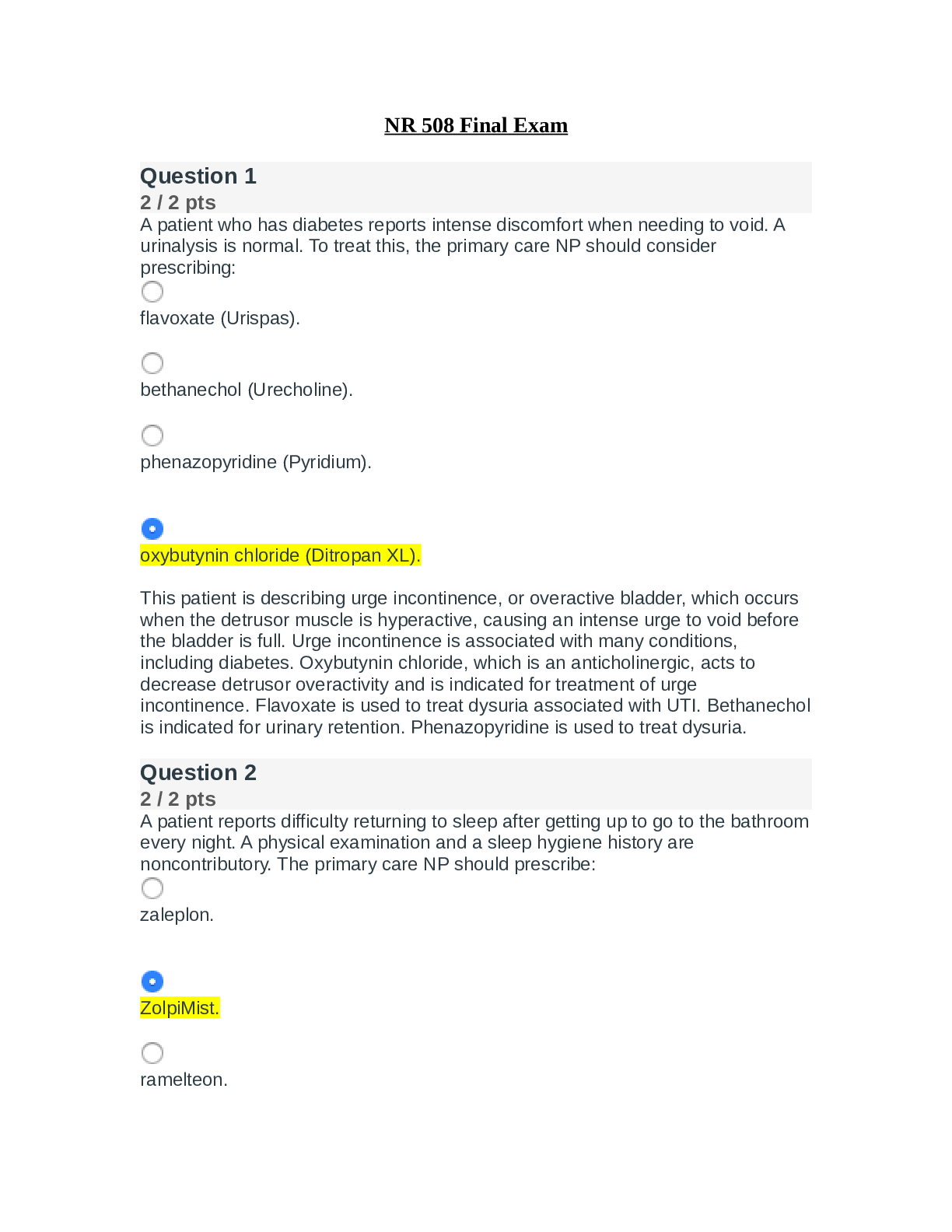
Experiment #6: Separation and Identification of an Unknown Binary Mixture Objective: To successfully separate and correctly identify the components of an unknown binary mixture using various Orga... nic Chemistry laboratory techniques. Introduction: Identity of unknown compounds is extremely vital for chemists. To ensure that they identify these unknown compounds there are several tests that can be performed. In this experiment a variety of organic chemistry methods were utilized to separate and purify an unknown binary mixture, and determine the compounds of the mixture. First the solid-liquid mixture under goes a basic vacuum filtration, and then each unknown is purified. Afterwards, an IR spectroscopy is performed for each unknown. Solubility tests and classification tests are performed for each to help determine what functional groups are present. Then, once the NMR data is obtained, one can begin to conclude the identity of obtained compounds, and set up a synthesis outline for derivatives. There are two different techniques are used to purify our unknown: recrystallization was performed for solid compound, simple distillation was performed for liquid part. The technique of crystallization is one of the most valuable available for the purification of solids. The basic idea of purification is easily understood and the manipulations are straightforward. In spite of this, crystallization remains more of an art than a science. A part of the trouble arises because a good crystallization frequently requires much patience. A more serious problem is that the best solvent to use cannot be chosen by a convenient magic rule, but must be found by trial and error. The most fundamental property of a good solvent for crystallization of a solid is that the hot solvent must dissolve the substance readily while the cold solvent must dissolve it sparingly. This means that we would start our hunt for a good solvent by looking for one that gave borderline solubility. From here we would have to adjust the temperature range, the ratio of solid to solvent, or try combinations of solvents to find a mixture with just the right solvent properties. In crystallization of a mixture of solids one is always faced with the practical problem of knowing how much solvent to use. If one of the components is poorly soluble in the chosen solvent one could go on adding the hot solvent for a long time before the mixture is dissolved completely. Distillation can be used as a method for purifying a single liquid and also as a means of separating a liquid from a dissolved solid or from a mixture of miscible liquids. The liquid (or mixture) is heated and when it boils, the vapors are condensed into a separate receiver. The resultant liquid (distillate) is collected in one or more fractions. A single liquid will begin to boil when its vapor pressure is equal to the vapor pressure of the atmosphere. For a pure liquid the boiling temperature should remain constant (within 2°C) for the duration of the distillation. Once the two unknowns are purified, the solubility tests, classification tests, IR and NMR can be performed to identify the compounds. Lastly, a derivative is formed for each unknown to verify that the correct identity is determined. A derivative is basically a new compound that formed from the unknown; however the main difference is that the derivative usually has a new functional group. After forming the derivative, the functional group test is performed and should be positive, whereas on the original unknown, it was negative. Also, the MP and if it is similar to the literature melting point of the derivative, then this would prove that a derivative was successfully created and the unknown was correctly identified. Methods/Procedure: Part A – Preliminary Examination Part B – Vacuum Filtration Part C – Recrystallization with melting point Part D – Simple Distillation Part E – Solubility Test (Experiment 55A, p. 45 [Show More]
Last updated: 1 year ago
Preview 1 out of 9 pages
.png)
Reviews( 0 )
Document information
Connected school, study & course
About the document
Uploaded On
Dec 13, 2022
Number of pages
9
Written in
Additional information
This document has been written for:
Uploaded
Dec 13, 2022
Downloads
0
Views
54


.png)
.png)

.png)
.png)














.png)