Chemistry > EXAM > SCIN131 Week 1 Quiz, 2021_ American Military University SCIN 131 W1 answered. (All)
SCIN131 Week 1 Quiz, 2021_ American Military University SCIN 131 W1 answered.
Document Content and Description Below
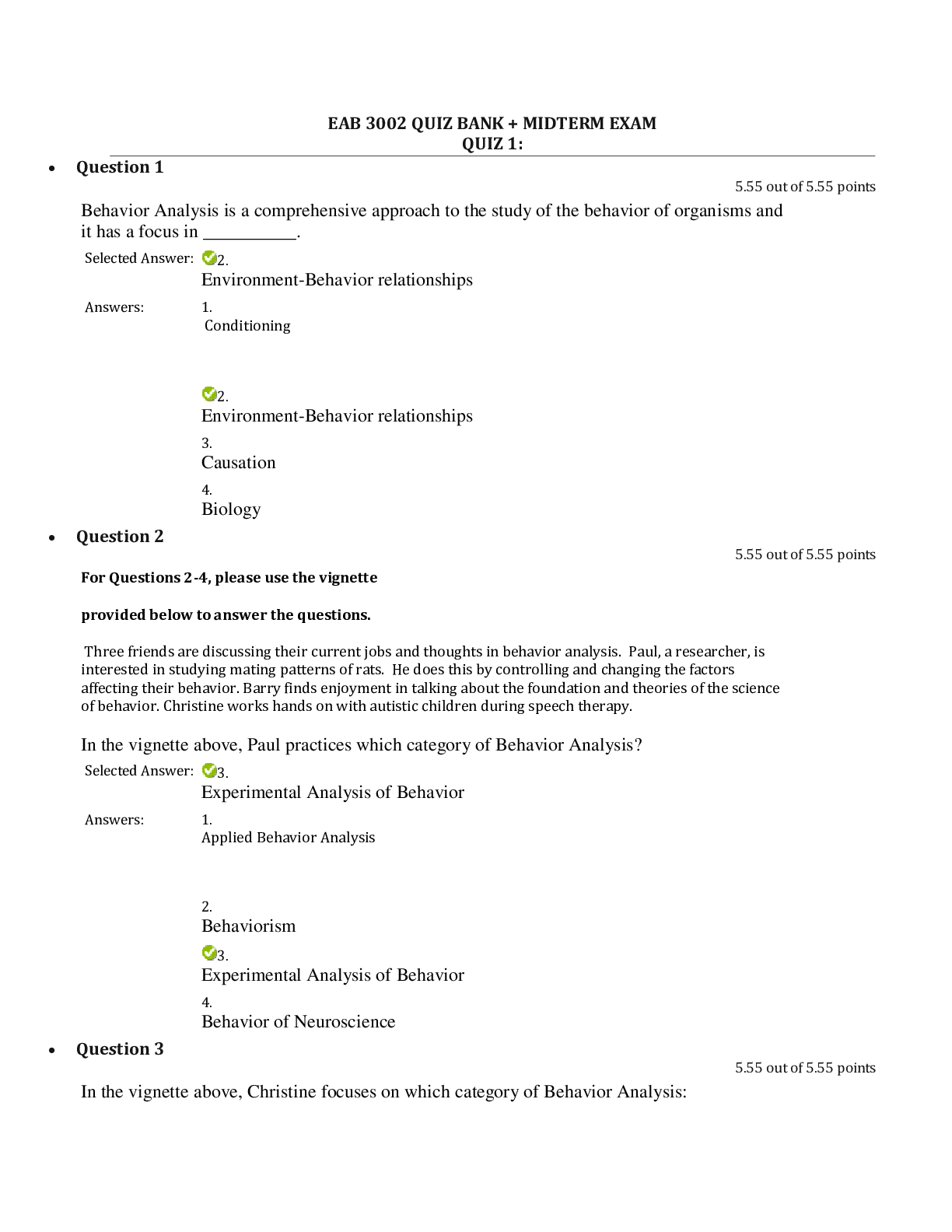
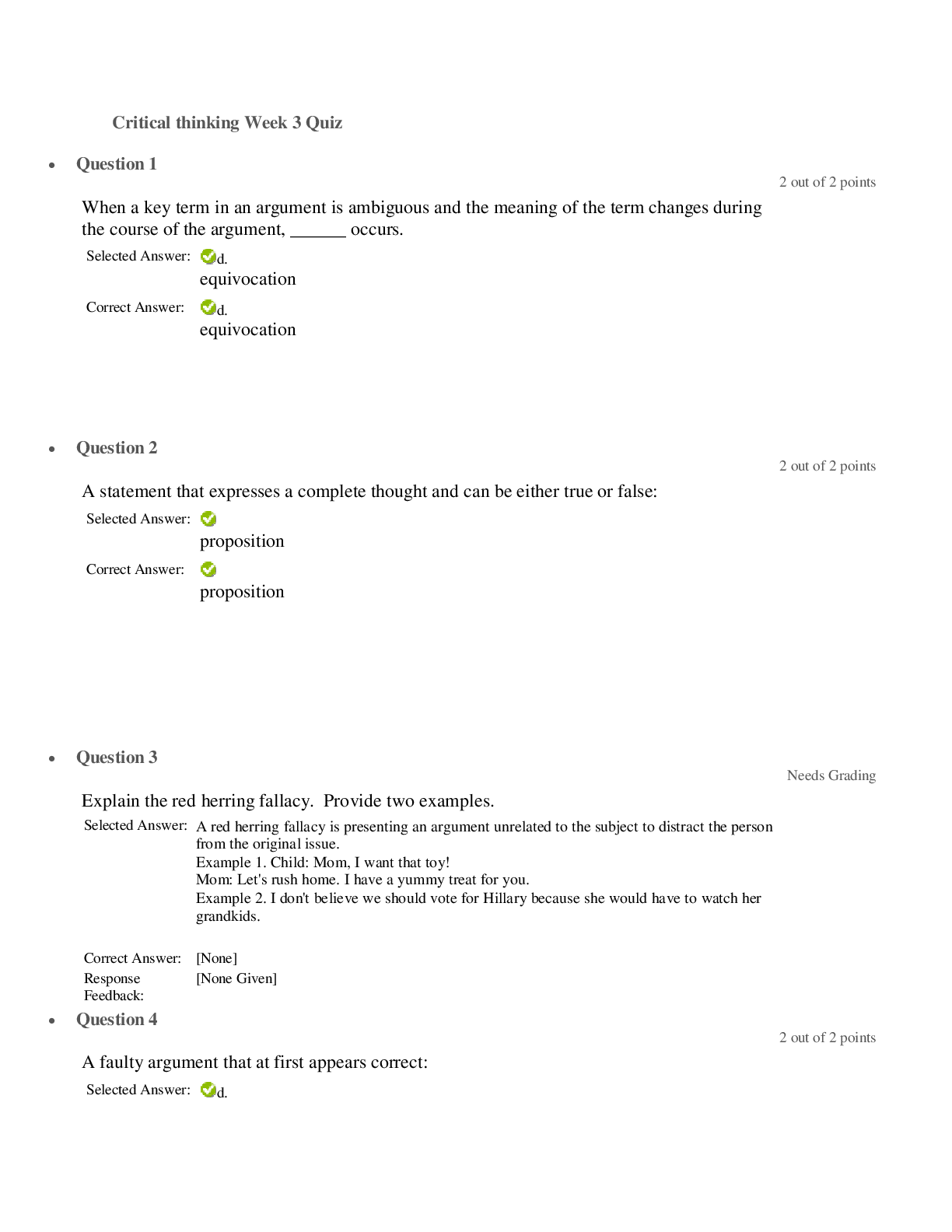
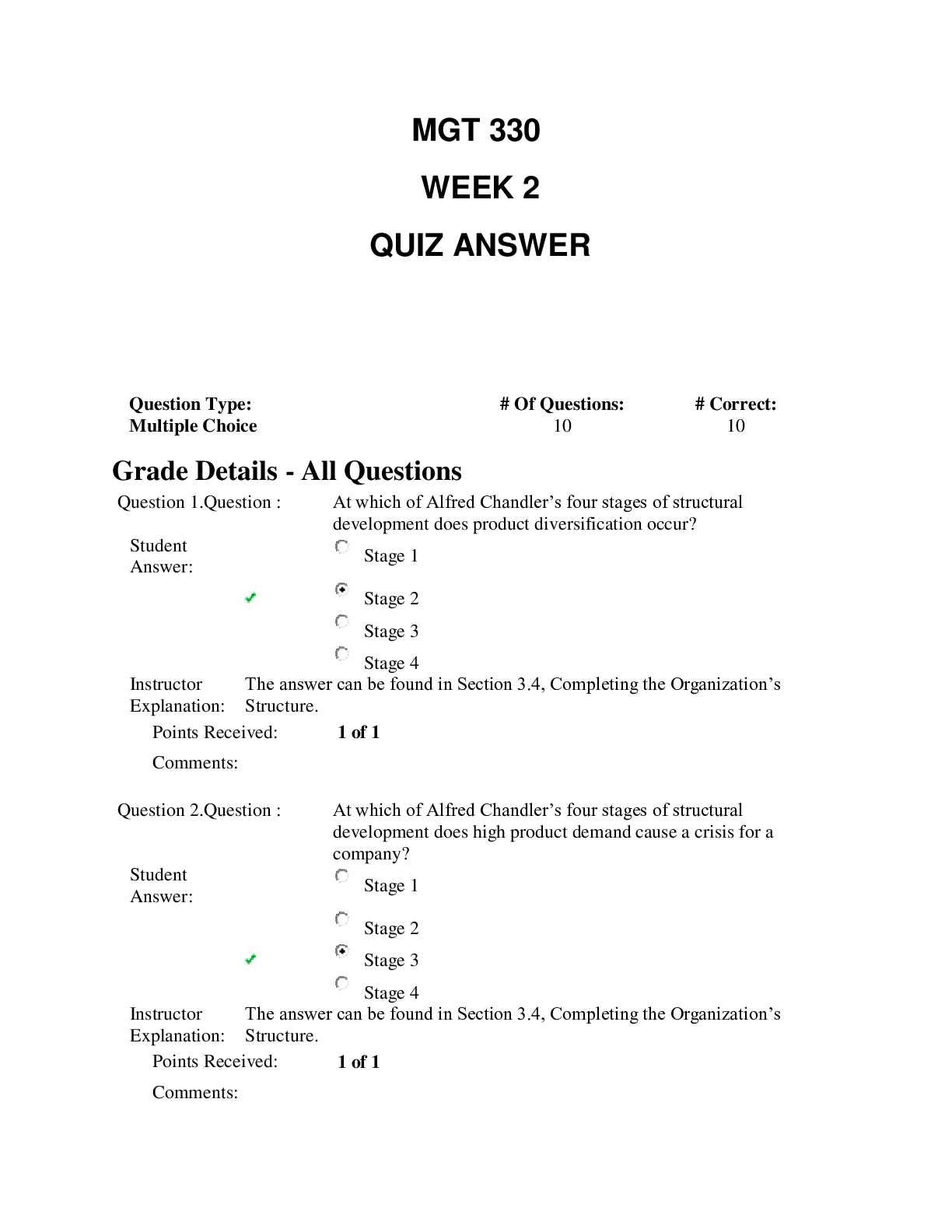
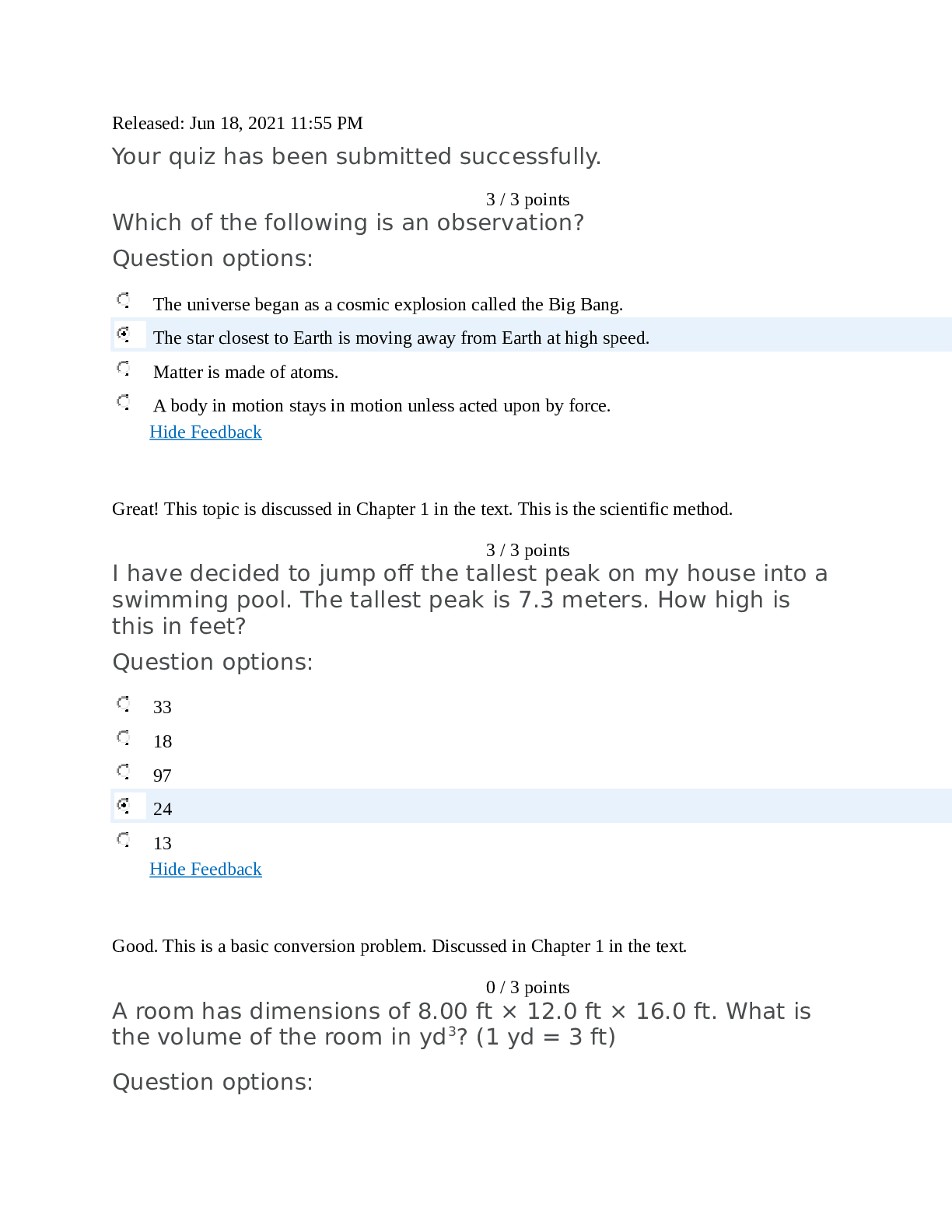
SCIN131 Week 1 Quiz. Released: Jun 18, 2021 11:55 PM Your quiz has been submitted successfully. 3 / 3 points Which of the following is an observation? Question options: The universe began ... as a cosmic explosion called the Big Bang. Matter is made of atoms. A body in motion stays in motion unless acted upon by force. Hide Feedback Great! This topic is discussed in Chapter 1 in the text. This is the scientific method. 3 / 3 points I have decided to jump off the tallest peak on my house into a swimming pool. The tallest peak is 7.3 meters. How high is this in feet? Question options: 33 18 97 13 Hide Feedback Good. This is a basic conversion problem. Discussed in Chapter 1 in the text. 0 / 3 points A room has dimensions of 8.00 ft × 12.0 ft × 16.0 ft. What is the volume of the room in yd3? (1 yd = 3 ft) Question options: 4.14 x 104 yd3 1.34 x 108 yd3 56.9 yd3 4.61 x 103 yd3 Hide Feedback This is a basic conversion problem, but with using exponents. Discussed in Chapter 1 in the text. 3 / 3 points The mass of a single copper atom is 1.055 × 10-22 g. This is the same mass as: Question options: g g g A sailor circumnavigated the earth and covered 4,264,000 meters. Express this number in standard scientific notation. Question options: 4.264 × 10-7 m 4.264 × 10-6 m 4.264 × 107 m Hide Feedback Great! Scientific notation is discussed in Chapter 1. 3 / 3 points For the number 204012.305, which of the zeroes are considered significant? Question options: Only the zero after the 2 Only the zero after the 4 Only the zero after the 3 Hide Feedback Great! Significant figures are discussed in Chapter 1. 3 / 3 points Over-the-counter ibuprofen pain medication typically contains 200 mg capsules. Which of these is the equivalent mass of ibuprofen in grams? Question options: 200 x 10-1 g 200 x 103 g 200 x 10-6 g 200 x 106 g Hide Feedback Good. This is a basic prefix conversion problem. This topic is discussed in Chapter 1. 0 / 3 points ' Which of these represents a physical property? Question options: n of grilled cheese (now that's a fun experiment!) rrosive) action of sodium hydroxide on aluminum dium chloride ove Which measurement of water below is equivalent to one cubic centimeter? Question options: 1 mL 1 cc 1 cm3 none of the above Hide Feedback Good. The topic is discussed in Chapter 1. 3 / 3 points How many significant figures will be in the final answer of this calculation? 2.15 × 11.2 ÷ 0.8765 = ________ Question options: 4 6 11 none of the above Hide Feedback Good! Significant figures are discussed in Chapter 1. Be sure to get these sig figs down at the beginning. Doing 3 / 3 points `Which of the following items is a mixture? Question options: bove Water is an example of: Question options: Round off 507,506 to three significant figures. Question options: 507 5.08 × 105 508 Hide Feedback Significant figures are discussed in Chapter 1. 3 / 3 points `How could one classify matter that has atoms close together but with an indefinite shape? Question options: bove `Which of the following is a heterogenous mixture? Question options: er Which of the following statements was not understood using Dalton's atomic theory? Question options: Molecules are formed from atomic combinations. Law of conservation of mass. Elements are composed of atoms. Hide Feedback This topic is discussed in Chapter 1 in the text. This is the history of chemistry. 3 / 3 points When the value 98.54 is rounded to two significant figures, the number should be reported as: Question options: 98 98.5 100 none of the above Hide Feedback Good. Rounding associated with Significant figures are discussed in Chapter 1. 3 / 3 points Convert 100 cm3 to m3. Question options: What are chemists most interested in? Question options: the study of life the study of the human body and how it is affected the science of matter and how it responds to the environment all of the above are things that chemists are interested in Hide Feedback Good! This is a basic inference based on the information given. 3 / 3 points A concise verbal or mathematical statement of a relationship between phenomena that is always the same under the same conditions is referred to as: Question options: a hypothesis. a theory. none of these. Hide Feedback Great! This topic is discussed in Chapter 1 in the text. This is the scientific method. 3 / 3 points `The correct number of significant figures in the number 0.000320 is: Question options: 7 4 ambiguous none of the above Hide Feedback Good. Significant figures are discussed in Chapter 1. 3 / 3 points Which scientist is responsible for determining that matter is neither created nor destroyed? Question options: Christian Doppler Gregor Mendel Louis Agassiz Charles Darwin Hide Feedback Correct! This scientist's work led to the principle in chemistry called Law of Conservation of Mass. 3 / 3 points Which of the following is considered "safe" laboratory behavior? Question options: Smelling chemical samples Wearing baggy clothing Wearing flipflops Being unorganized Hide Feedback Correct! It is important to follow all lab safety rules to avoid accidents and injuries. 3 / 3 points Which of the following principles is important to drawing scientific conclusions? Question options: Subjectivity Opinion Imprecision Bias Hide Feedback Correct! Conclusions about scientific phenomena can be drawn when multiple scientists can reliably repeat the s 3 / 3 points Shake laboratory thermometers down before use. Question options: True Hide Feedback Watch the ACS safety video. 3 / 3 points Remove rings and watches before working in the laboratory. Question options: False Hide Feedback Watch the ACS safety video. 3 / 3 points If you don't have an inserter, lubricate glass tubing before inserting it through a stopper. Question options: False Hide Feedback Watch the ACS safety video. 3 / 3 points Wear closed leather shoes to protect your feet while in the lab. Question options: False Hide Feedback Watch the ACS safety video. 0 / 3 points Carefully scoop up mercury from a broken thermometer with a piece of paper. Question options: False Hide Feedback Watch the ACS safety video. 4 / 4 points How do you add the water when diluting acid? Always add acid to water. Slowly add the acid to the water using a funnel and slowly mixing it. (water acts as heat). 4 / 4 points How much of your clothing should you remove on the way to the safety shower? You should try to remove all clothing prior to getting to the safety shower due to the fabric of the clothing being contaminated and causing more harm or injury. 5 / 5 points In 2-3 sentences, what was the overall purpose of the lab for this week? The overall purpose of this weeks lab was to demonstrate the importance of safety when it comes to working with chemicals in a laboratory setting. We also learned the basic knowledge of certain chemicals, how to convert to meters and yards, what significant figures are. 85 / 100 - 85 % 85 / 100 - 85 % [Show More]
Last updated: 1 year ago
Preview 1 out of 13 pages
Reviews( 0 )
Document information
Connected school, study & course
About the document
Uploaded On
Jun 28, 2021
Number of pages
13
Written in
Additional information
This document has been written for:
Uploaded
Jun 28, 2021
Downloads
0
Views
89