Physics > Experiment > GIZMOS.CHEM 102 Student Exploration: Photoelectric Effect (All)
GIZMOS.CHEM 102 Student Exploration: Photoelectric Effect
Document Content and Description Below
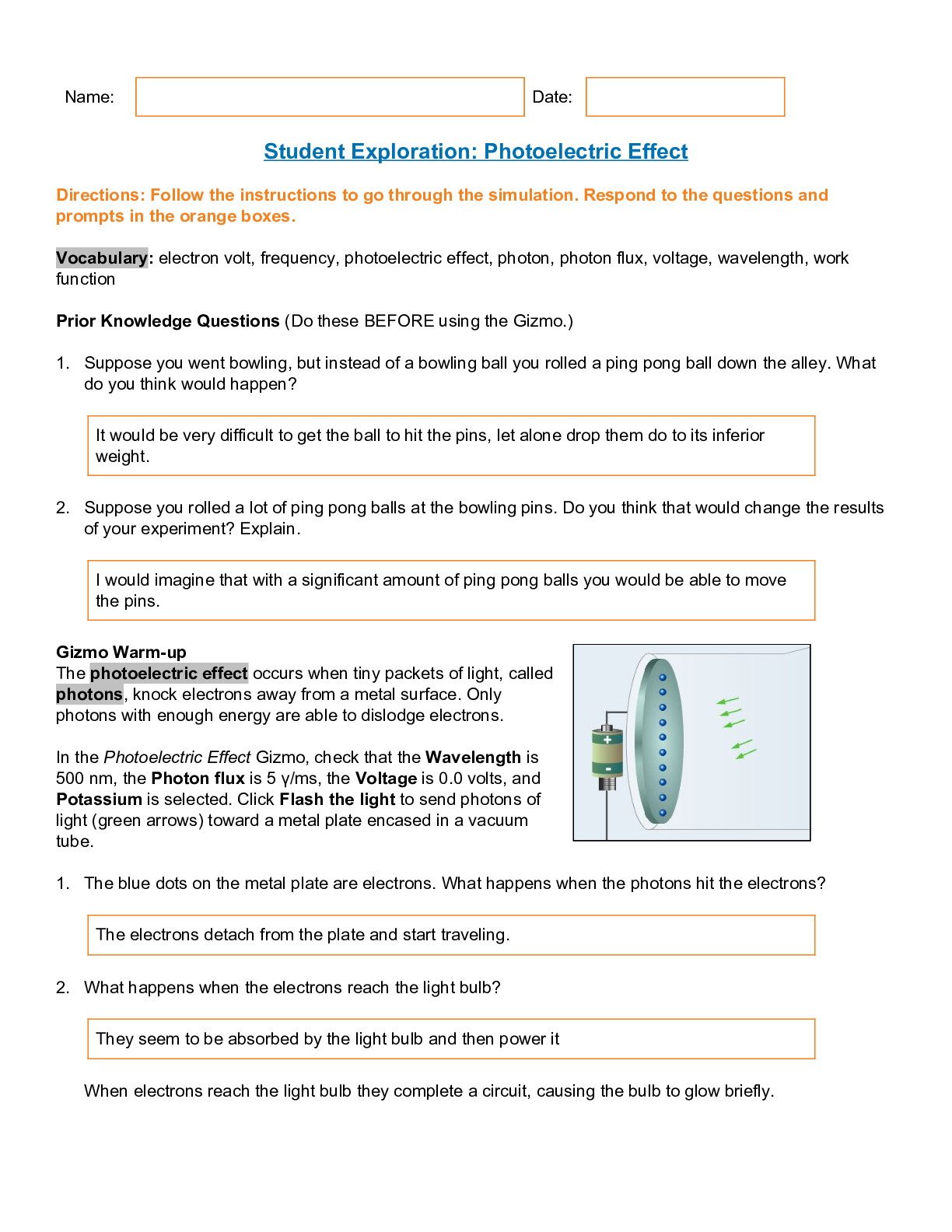
Student Exploration: Photoelectric Effect Directions: Follow the instructions to go through the simulation. Respond to the questions and prompts in the orange boxes. Vocabulary: electron volt... , frequency, photoelectric effect, photon, photon flux, voltage, wavelength, work function Prior Knowledge Questions (Do these BEFORE using the Gizmo.) 1. Suppose you went bowling, but instead of a bowling ball you rolled a ping pong ball down the alley. What do you think would happen? 2. Suppose you rolled a lot of ping pong balls at the bowling pins. Do you think that would change the results of your experiment? Explain. Gizmo Warm-up The photoelectric effect occurs when tiny packets of light, called photons, knock electrons away from a metal surface. Only photons with enough energy are able to dislodge electrons. In the Photoelectric Effect Gizmo, check that the Wavelength is 500 nm, the Photon flux is 5 γ/ms, the Voltage is 0.0 volts, and Potassium is selected. Click Flash the light to send photons of light (green arrows) toward a metal plate encased in a vacuum tube. 1. The blue dots on the metal plate are electrons. What happens when the photons hit the electrons? 2. What happens when the electrons reach the light bulb? When electrons reach the light bulb they complete a circuit, causing the bulb to glow briefly. Name: Date: It would be very difficult to get the ball to hit the pins, let alone drop them do to its inferior weight. I would imagine that with a significant amount of ping pong balls you would be able to move the pins. The electrons detach from the plate and start traveling. They seem to be absorbed by the light bulb and then power itIntroduction: Through the centuries, many scientists have debated whether light is a wave or a stream of tiny particles. In the 1800s, most scientists agreed that phenomena such as refraction and diffraction supported the “light as a wave” theory. However, Albert Einstein’s explanation of the photoelectric effect showed that light can act like a stream of particles as well. Question: What factors affect the ability of light to free electrons from a metal surface? 1. Observe: Click Flash the light with a variety of wavelength values. What do you notice? 2. Observe: The photon flux is a measure of how bright the light is. It is equal to the number of photons that are released in a given time. It is given as photons (γ) per millisecond (ms). Click Flash the light with a variety of Photon flux values. What do you notice? 3. Form hypothesis: Answer the following questions based on what you have observed so far. 4. Investigate: Set the Photon flux to 1 γ/ms. Use the Gizmo to find the longest wavelength that will dislodge an electron from the metal surface. What is this wavelength? 5. Predict: Set the Wavelength to 540 nm. What do you think will happen if you flash the light with a photon flux of 1 γ/ms? What if you flash the light with a flux of 10 γ/ms? [Show More]
Last updated: 1 year ago
Preview 1 out of 6 pages
Reviews( 0 )
Document information
Connected school, study & course
About the document
Uploaded On
May 30, 2021
Number of pages
6
Written in
Additional information
This document has been written for:
Uploaded
May 30, 2021
Downloads
0
Views
106










.png)

.png)





