Chemistry > Lab Report > Lab Report #7 SN2 1-bromobutane San Antonio College CHEM 2423 (All)
Lab Report #7 SN2 1-bromobutane San Antonio College CHEM 2423
Document Content and Description Below
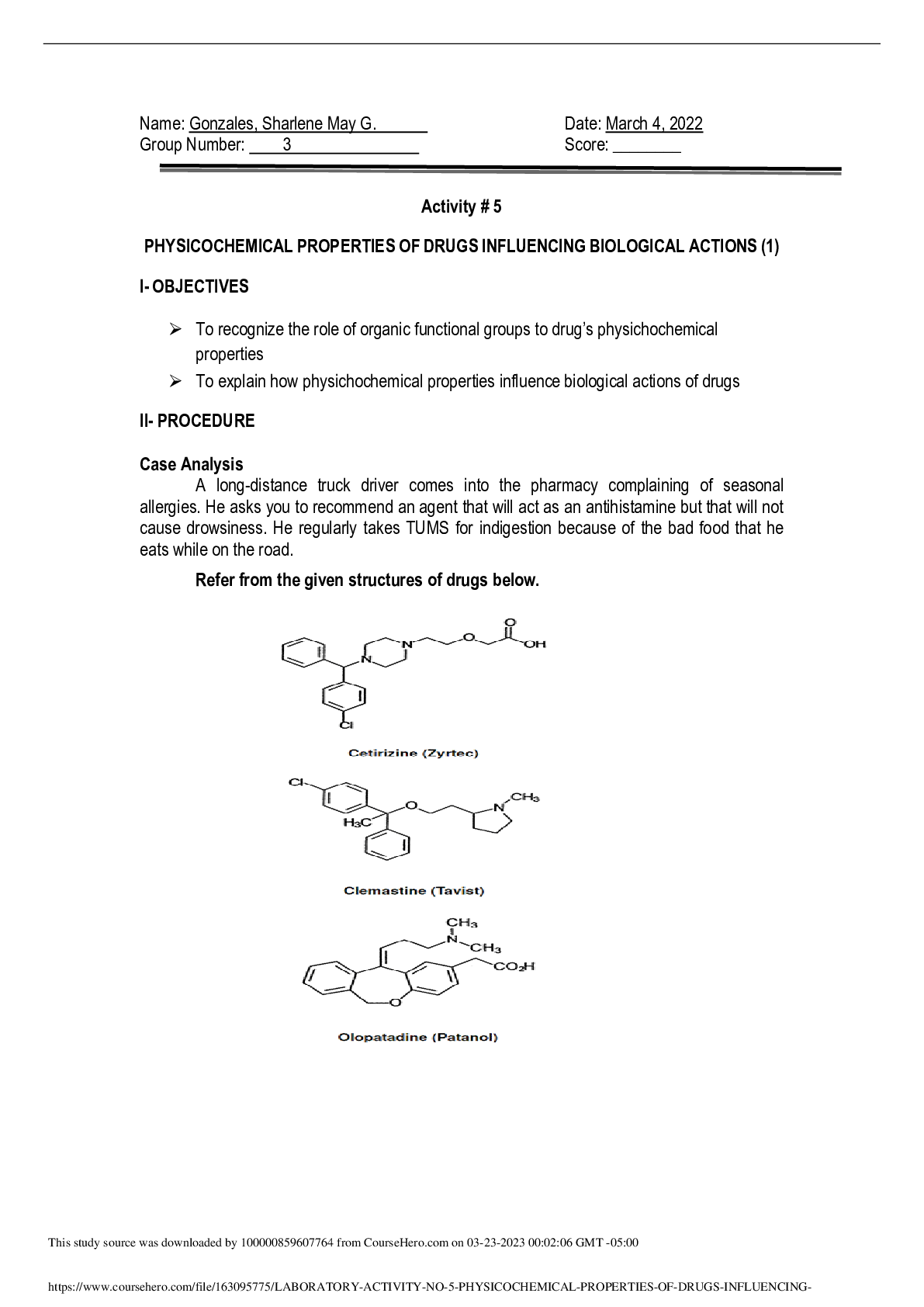
Title: Preparation of 1-bromobutane; An SN2 Reaction Abstract: A substitution nucleophilic bimolecular reaction was carried out utilizing 1-butanol and sodium bromide in the synthesis of 1-bromobut... ane. Reflux, separation, washing, and simple distillation were used to separate andn purify 1-bromobutane. No distillate of 1-bromobutane was collected, and is pending H-NMR analysis to confirm identity. Introduction: Bromination of butane through a radical reaction would undergo halogenation at a second degree carbon, yielding 2-Bromobutane as its primary product. This phenomenon could be explained by the lower energy transition state in the bromination of a secondary substrate. For the production of 1-Bromobutane then a more complex process must be followed to accomplish a halogenation at a primary carbon. The nucleophilic substitution of 1-butanol allows for the halogenation at carbon 1 through an SN2 reaction. First Water, Sodium Bromide, 1-Butanol and Sulfuric acid were mixed and by method of reflux the product 1-Bromobutane could be able to be retained within the reflux system, by condensation. The mechanism of this reaction is a substitution by a nucleophile bi molecular reaction, where first the alcohol functional group is protonated by the sulfuric acid, and converted into a better leaving group so that the SN2 reaction then can take place. The bromide ion acts as the nucleophile, attaching the primary carbon and causing the water molecule to leave in one concerted step. In this reaction, the formation of HBr is is also produced as a byproduct due to the 1-Butanol being the limitant reactant, thereby leaving excess bromide molecules forming HBr acid. Due to the gaseous state of HBr, it was easily aspirated into the NaOH flask where it underwent an acid base neutralization reaction forming a NaBr salt and water. After a period of about 30 minutes of refluxing to take place, the product 1-bromobutane was separated using a separation funnel, washed to remove any residual 1-butanol, with sulfuric acid, Naoh and Water respectively. Specia [Show More]
Last updated: 1 year ago
Preview 1 out of 3 pages

Reviews( 0 )
Document information
Connected school, study & course
About the document
Uploaded On
Nov 20, 2022
Number of pages
3
Written in
Additional information
This document has been written for:
Uploaded
Nov 20, 2022
Downloads
0
Views
77





.png)








 (1).png)






.png)

.png)

