Pharmacology > Class Notes > oral penicillins (All)
oral penicillins
Document Content and Description Below
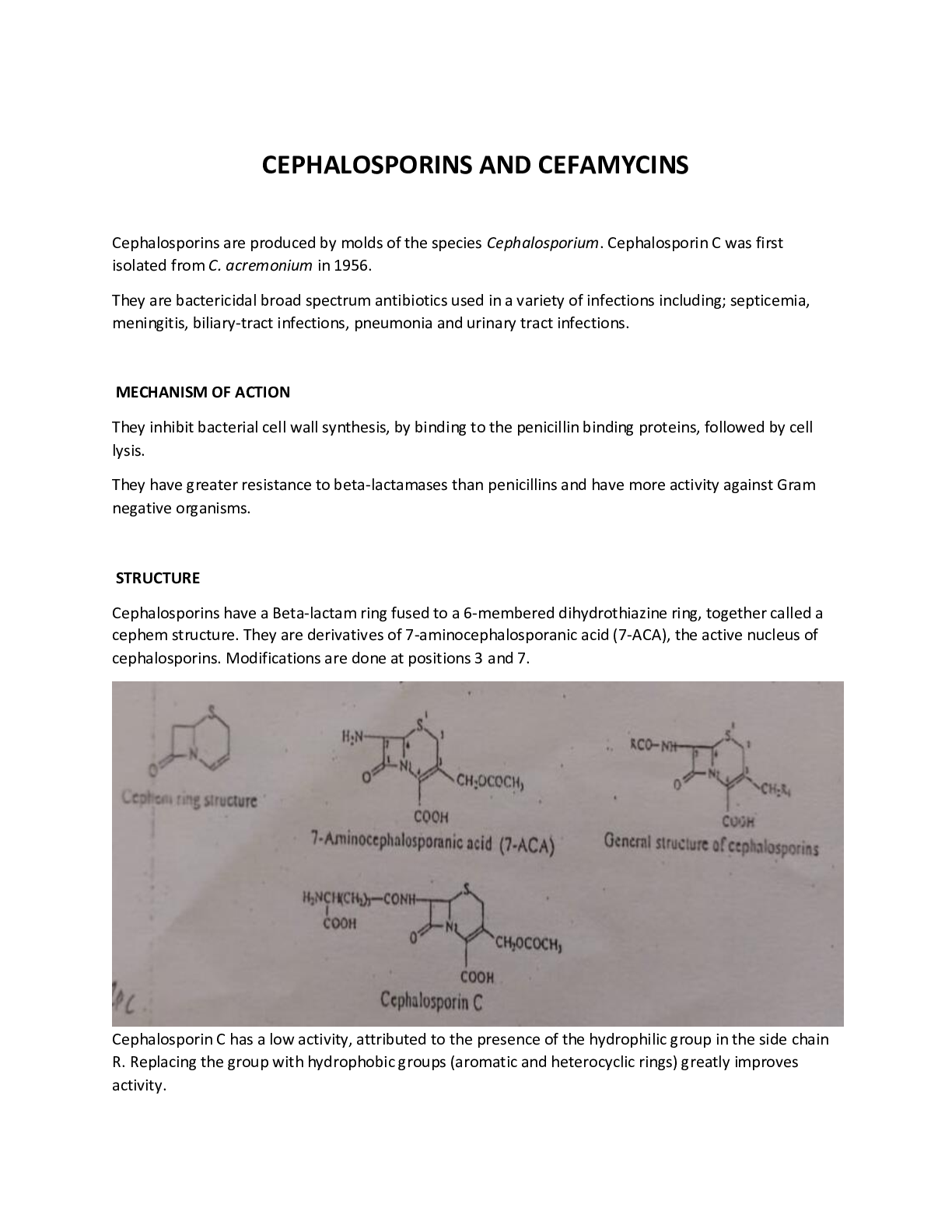
ANTIBIOTICS (i) Oral penicillins Phenoxymethy penicillin • Produced by penicillium chrysogenum • Given orally • fairly well absorbed in GIT • Can be in the form of K+. Known as co... mpocillin or Bezathine salt known as a pen V suspension • Was the 1st oral penicillin Others – amoxicillin, ampicillin, cloxacillin, flucloxacillin. (ii) To prolong duration of action You can either delay the absorption so that it is absorbed for long hours or delay the elimination. Delay absorption Substition of Na+, K+ with more insoluble salts eg a procaine moiety, benzathine, Benethidine. The absorption is slow for procaine and more slow for Benzathine eg Benzathine penicillin you can have a blood concentration, for 1 wk after a single 1m injection. Procaine penicillin is absorbed slowly. The absorption can be delayed further by formulating it in an oily preparation 3000 iu (aq) - 12-24hrs 3000 iu (oil) - 24-48hrs 3000 iu (oil) + aluminium stearate – 48-96hrs depository preparations When a patient is very sick, you give a penicillin which is absorbed fast then you give another which is absorbed slowly so that the blood concentration, is maintained for a long period. sodium pen G procaine pen G benethidine pen G bld conc time PPF (portified procaine penicillin.)it is fortified to give it a fast blood concentration and then procaine delays its absorption . Delay the excretion • Give with probenecid eg for treatment of gonorrhoea 3g amoxicillin + 1g pobenecide Other drugs delay the excretion of penicillin eg aspirin, indomethacin. (iii) Penicilinase resistant penicillins The 1st penicillinase resistant penicillin was methicilin The current ones in use: • Cloxacillin • Flucloxacillin) • Dicloxacilin • Dioxacillin • Quinacillin • Nafcillin • Diphenicillin Iv Broad spectrum penicillin Bacteria have developed resistance against ampicillin Esters of ampicillin are given because they are better absorbed than ampicillin eg: • Pivampicillin • Bacampicillin • Talampicillin Well absorbed in blood and give a higher blood concntration. Pondocillin, amboxin are common in the market. The esters have not been detected in bld. Amoxicillin is broad spectrum Broad spectrum penicillins have been misused. (v) Penicillins effective against preudomonas aeruginosa • Carbenicillin • Ureidopenicillins – Aziocillin & Meziocillin (Byopen) • Piperacillin • Ticarcillin • Calindacillin • Carfenicillin (vi) Combine β lactamase inhibition + Penicillin Potassium clavulonate + Amoxicillin – Augmentin Clavulonic acid protects amoxil from breakdown by bacteria Picarcillin (3g) + Clavulonate (200mg) – Timentin Ampicillin + Salbactam Piperacillin + Tazobactam Shortcomings such as allergy have not found a solution but different antibiotics are used. Today combination of penicillin is important eg Ampicillin + cloxacillin - ampiclox This increases the sensitivity. Cephalosporins 1st Generation cephalosporins: The antimicrobial spectrum was very close to that of pencillin . (G +ve) They include: • Cefadroxyl – • cefazolin • cephalexin (reflex) • cephalothin • cephapirin • cephadine 2nd generation of cephalosporins, Mostly against (G-ve): eg • cefaclor • cefamandole • cefanilia • cefuroxime • cefprozil These did not work effectively on the G –ve. The search continued. 3rd generation cephalosporins • ceftazidime (fortum) • ceftriaxone (rocephine) • cefotaxime • ceftixizone Have to be given – parenterally 4th generation cephalosporins Eg cefepime Not very distinct Have extended microbial spectrum May have other activities eg activity on pseudomonas aeroginosa Recent development emphasizes on: • Development of extended microbial spectrum • Drugs which can be given orally • Long duration Clinical use The cost is very important, despite being very effective If a patient is allergic, they are not used. 9% of patient allergic to penicillin show cross allergy to cephalosporin. Allergic reactions such as urticaria, angioedema, anaphylaxis should be avoided. Very few bacterial infections have cephalosporin as the antibiotic of first choice. MACROLIDES They were discovered to solve problems of penicillin such as allergy It is a large group of closely similar antibiotics mainly discovered 1950-1954. All of them have a large structure ie macrocyclic structure with 13-16 C They have a unique N containing sugar, hence K a macrolide and MW is large - >700 They are weak bases slightly soluble in H2O but freely soluble in organic solvents They are regarded as alternatives to penicillins Mostly against G+ve Broad spectrum Mostly against G-ve Penicillin Tetracyclines Aminglycoside Cepharosporins Chloromphenical Fluoroquinolone Macrolides Other penicillin Their Antimicrobial spectrum is mostly effective against the G+ve eg Strep. Cocci. If a person cannot be given penicillin, Macrolides are given. Examples: • Erythromycin • Clarithromycin • Azithromycin • spriamycin • Josamycin – Japanese • Rosamycin – Japanese Erythromycin Used extensively It was discovered in 1952 by Lily Research Labs by Mcguire It is isolated from streptomyces erthreas. It has 3 components ie erythromycin A,B & C but erythromycin A was discovered to have significant activity so B& C were ignored. Mode of Action Inhibit bacterial protein synthesis. They have no effect on cell wall synthesis. React with ribosomal processes interfering with incorporation of some amino acids eg Phe. Stability Unstable below pH 5 hence given as enteric coated tablets for base or can be given as esters. They are better absorbed as esters. Antibacterial spectrum Is similar to that of penicillin. Are also effective against N. Gonorrhoea, Brucella pertusis Drug of choice in Treponama pallidum Not used for rxn of GIT microorganisms (G-ve). The absorption is very poor and it icannot pass through the cell wall. Not need for fungalinfections. Very effective for respiratory infections. Absorption and distribution • It is readily absorbed for the upper part of the small intestines. It is taken up by tissues and the concentration in tissues remain high than in blood. • Given as enteric coated or ester stearate salt. • It can also be given as an injection. Peak blood level is after 1-4 hours. Excretion Mostly excreted through bile in an active form therefore has been used to treat billiary tract infections. It is excreted in faeces thus can Kill the GIT bacteria causing super infection. Some comes in urine therefore used in syphilis. Resistance No resistance to staph. Aures, strep.pneumonia, pyrogenes etc. Resistance is noted after prolonged use Toxicity Erythromycin esterate was found to cause liver damage. Other salts are used. It causes nausea and GIT disturbance, promethazine, Metodopramide, benzodiazepins can be given to patients who are vomiting. Clinical use Used as an alternative to penicillin in patients allergic to penicillin Also useful for penicillin resistant microorganisms. This is not the case today because there are many alternatives. Used for treatment of respiratory infections in children especially below 5 years. Useful in mycoplasma pneumonia In Adults the response to erythromycin in respiratory infections is not good. Clarithromycin A derivative of erythromycin (6-0 methyl) The antimicrobial spectrum is similar to that of erythromycin. (For Acute and community acquired infections such as Strep & Staph cocci) It is effective against myc. leprae, myc avium. This bacteria is found mainly in HIV patients. It may not be used clinically for treatment of leprosy Useful in treatment of toxoplasmosis. Azithromycin Effective for respiratory tract infectious Classified as broad & medium spectrum It is widely distributed throughout body tissues The level in tissue is 50 times that of plasma It is tissue bound. Target tissues are lungs, prostate, tonsils It exceeds the MIC 90% of mostly microorganisms Given 500mgOD 3/7 or 500mg stat then 250mg Effective against Myc. avium Spiromycin Isolated from streptomyces Has very low invitro activity compared to erythromycin Advantage is that it persists in tissue for a long time after blood concentration have gone down it is effective against staphylococci sepsis. AMINOGLYCOSIDES The aminoglycosides have the a similar structure characterised by a large amino sugar linked to another moiety known aminocyclitol. Antibacterial spectrum Are potent bacteriacidal agents In general they are active against G-ve bacteria E-coli, Krebsiella, Shigella, N. gonorrhoea. Are also effective against many G-+ve bacteria eg Staph, Strep Cocci. The aminoglycosides are active against My.tuberculosis (1g 1m daily for 8 wks). Pharmacokinetics They are very similar They are very polar therefore poorly absorbed from the GIT when given IV, they can therefore can used to sterilize the GIT eg prior to surgery The drug is excreted unchanged in faeces. Are given IV, 1m etc. Distribution: Diffuse fairly rapidly into the body tissues CSF, Peritoneal, pleural fluids. Because they are polar, they are rapidly excreted therefore given frequently. Can also be used to treat infections in urine because they are excreted unchanged. Where the renal function is compromised, the toxicity signs may show . Elimination is very variable. Excretion is primary by glomerular filtration although small part is accounted for by active excretion. Bacterial resistance It is common and rapid with many bacteria. Are combined with other eg in treatment of TB. Resistance is quite significance. Toxicity: Quite common Damage the 8th cranial nerve leading to deafness, vesticular disturbance, circling movement Nephrotoxicity is common particularly with some members of the group Hypersenactivity non is common eg fever ashes, fever. Skin sensitization is common among pharmacists and nurses. Pressure neuromuscular blockage. Clinical use In treatment of TB eg streptomycin. Other can also be used. In treatment of plague streptomycin is the drugs of choices Pseudomanas infection – Gentamycin is very applicable but other drugs may be used. gentamycin is still used for skin infections eg in burns, corynbacteria and other G-ve bacteria. Gentamycin is often used in combination with the β-lactams especially Benzylpenicillin: • Gentamicin • Pen G • Metronindazole There is synergism between gentamicin and pen G used in enterococcal carnditis. MOA Binds to the 30s and 50s submit and interferes with protein synthesis. Streptomycin Usually streptomycin sulphate is soluble in H2O. It is used as a base or salt It is stable between pH 3-10 and below 18oC. It is supplied as sulphate to reduce pain and irritation at site of injection. Antimicrobial spectrum is typical of the group Effective in my tuberculosis, staphy cocci. It is bacteriacidal operating at a cone higher than the MIC. Bacterialstatis Bacterioidal MIC It is non-ionized at pH 8 Resistance is a very serious. Occurs within a few days in TB treatment For TB it has to be used in combination with the drugs ADME is typical of the group. Excretion is by glomerular filtration (40-90 %). Given 1m. Clinical application Used mainly in TB therapy Also the drug of choice in plague, brucellosis. Used in some urinary infections. Hypersensitivity Rxn and pain at infection sites make it not to be used a lot. Neomycin Not used currently. Like the others in spectrum pharmacokinetic properties Clinical use Antibiotic powder has neomycin as one of the 3 antibiotics in glabbacin. In eye drops N. Also component of ear drops. When it was used it was combined with other antibiotics eg in antibiotic powder polymyxin, bacitracin used topically for local treatment of infections in combination with others. Gentamicin There are several compounds under it. Gentamicin C, split to C 1A &C2. Typical antimicrobial spectrum is typical of the group. Much more potent; the efficacy is higher than for streptomycin therefore low dose(40mg/ml, 80mg/ml.) Active against Ps,aeroginosa x10 than streptomycin. Resistance is very common in pseudomonas, streps, staphs etc. ADME is typical: excreted unchanged in urine crosses the placenta. Toxicity – damages the 8th cranial nerve leading to vestibular disturbance. And otototoxicity General purpose Useful in coli form infections which are life threatening at various sites. In UTIs pseudomonas A. used alone or can be used in combination with other antibiotics Useful for blood septicimea (G –ve) Useful in severe sepsis following abdominal surgery Drug is applied locally to eliminate highly sensitive staph. infections on burns as a 0.3% cream ointment or solution Could be used in menengitis caused by gram -ve bacteria. Commonly combined with β-lactams. . Kanamycin A mixture of 3 antibiotics (aminoglycosides) – kanamycin A,B&C The active compound is kanamycin A Antibacterial activity is typical Active against myc, tuberculosis Resistance is very common – staph, enterobacteria. Excreted by kidneys Toxicity: 8th cranial nerve damage & Nephrotoxicity Modified kanamycine eg tobramycin It is deoxykanamycin B.which is 4 times more active than gentamycin. Its more active than .More active against pseudomonas. Active against several bacteria resistant to gentamycin. Similar to gentamycin and is mostly used therefore can be used for the same purposes. It is more expensive than gentamycin. Chloxamphenical Was discovered in 1947 from a soil sample in venenzuela streptomyces venenzuela. Another group was also isolated it in 1948. Shares another distinction – was the 1st antibiotics to be produced synthetically. There are four isomers of chloramphenical but none has greater activity than the original chloramphenical -yellow crystals -very bitter taste except chloramphenical palmitate which is tasteless Stable to boiling Antimicrobial spectrum Broad spectrum. Active against G+ve and G-ve bacteria Three are particularly sensitive: Salmonella typhi Bordetella pertusis Haemophilus influenza It is bacteriastatic and bactericidal to certain bacteria e.g H. Influenza, N. Gonorrhoea Mode of action Interferes with protein synthesis by combining with the 50 s subunit Resistance Chloramphenical is not very widely used so resistance is minimal. There are resistant strains of N.gonnorhea, H. Influenza etc Pharmacokinetics Well absorbed on oral administration Bioavailability is dependent on the dissolution rate There is variation in blood levels depending on the product used Parenteral solutions are available as chloramphenical sodium It is well distributed in blood. About 60% is bound to tissues. Highest in kidney>liver >lung>spleen>muscle>brain It penetrates part of the eye including the aqueous humour It is fairly well absorbed and reaches the CSF with a concentration , much higher than for many other antibiotics therefore used in meningitis. Found in glandular secretions. Metabolized before excretion by glucoronide formation and certain other higher amines. Metabolism occurs in the liver. It is known to inhibit hepatic microsomal mixed function oxidases. Interaction with talbutamide, dicumarol is possible (Phenyton, chlorpropamide) Excretion –is Mainly through the renal route. Appropriately 90% is recovered in urine with only 10% of this in free form. Some 3% is recovered in bile. In very young infants, the glucoronyl transferase metabolism is hindered and the glomerular tubular excretion is low and accumulation occurs in blood. SE and toxicity 1. It is not safe; it may cause a plastic anaemia. It is a potential bone marrow depressant. Granulocytopenia is not seen so total aplasia of the bone marrow results. The incidence 2 in a lot 1 million people. Depending on the dose can be 8 in 1m therefore the chances of aplasia are remote. 2. Gray baby syndrome associated, with the inability of the neonates to metabolise chloramphenicol. It is charactersed by vomiting, refusal to suck, abdominal distention, circulatory collapse with flaccidity ashen colour. Hypothermia also results. It is attributed to plasma level of chloramphenicol in the neonate. Neonates are not able to metabolise it. 3. Visual disturbance – blurred vision 4. Nausea 5. Vomiting 6. Unpleasant tasle 7. Diarrhoea especialy in patients being treated for typhoid to the high dose. Phenobarbitone, or diazepam can be used to overcome the symptoms. 8. Encephalopathy which is non specific. The patient is half conscious. This is due to phenylamine depletion. Chloramphenical is thought to interfere with the phe. Clinical uses Used for major infections and should be avoided for minor infections. • Typhoid fever – • Other salmonella infections • Ear and eye preparations as a solution • For meningitis • Severe respiratory infections are to H. infleunzae. In B.pertusis, it gives good results in severe cases but should be started early. max 2g in a day for 5 days is given • can be used in anaerobic infections eg instead of clindamycin especially for brain abscess. Here it can be used in combination with penicillin. • Can also be used in intraabdominal pelvic absences. It can replace clindamycin and metranidazole Thiamphenicol It is less active than chloramphenical against many G+ve and G-ve bacteria. It has no advantage over chloraniphenicol. It is not a substrate of glycoronyl transferase therefore excreted in urine in the active form,. rarely in the Kenyan market. Sulphonamides Sulphacetamide is a sulphonamide used as eye drops. Its sodium is neutral Its sodium is neutral therefore does not cause irritation. Metabolism Acetylation .The resulting product is very insoluble and places in the kidney to cause crytaluria Metabolism depends on individual and species eg in dogs there is no acetylation. Toxicity They are considered to be displacement drugs eg A pt who is already on walfarin. If a sulphoramide is administered warfarin is displaced to a cause a situation similar to overdose. They also displace penicillin for being bound causing synergism. Bilirubin is protein bound and sulphonamides can displace it. This not very significant in an adults but in newborns it may have serious repercussions.. Bilirubin when displaced goes to brain to cause brain damage –( Kernicterrus). should not be given to newborns, pregnant women. Renal toxicity The acetylated sulphonamide precipitates in the kidneys and causes hematuria. Some of the sulphonamides responsible are sulphadiazine, sulphamerazine, sulphamethazine, sulphapyridine. They are ka, the pyridine congenors. The extent of precipitation is depended on: • Intrinsic solubility • Degree of acetylation • PH of urine – PH fluctuates oftenly • Rate at within excretion is taking place. Rapid excretion causes a more likelihood of prepitation taking place therefore the very long acting sulphonamides do not pose this problems. Crystaluria can be eliminated by: Using sulphonamides within are more soluble Using sulphonamides within are excreted slowly Telling the patient to take a lot of water. Sometimes patients are given the sulphonamides with sodium bicarbonates • Use of triple sulphonamides eg sulphathiazole – 37% sulphadiazine 37% sulphamerazine 26%. The solubility of each sulphonamide is dependent on each other. Hypersensitivity: This is because they are protein bound. This is serious problem with the long acting sulphonamides. The drug protein complex is antigenic and antibodies are formed. Reactions take the form of drug fever, dermatitis etc. • Stevens Johnson syndrome – Inflammation on the lips and face. It is rare but fatal. Also affects skin, mucous membranes, conjunctivitis • Mild bone marrow depression – agranulocytosis, a plastic anaemia etc. Clinical uses For every sulphonamide sensitive infection there is an antibiotic. Sulphonamides are not very potent. The are not being used as independent but as combinations with trimethoprim eg cotrimoxazole which has a broad spectrum and used especially for respiratory tract infections and urinary tract infection. T They are cheap. Easy to administer :given orally. Are used for meningococcal meningitis eg sulphadiazine is very useful for this. NITROFURAN DERIVATIVES Tend to have a broad spectrum. Effective against g +ve, g-ve, several protozoa and fungi. Nitrofurantoin is used for urinary tract injections. Nifuradene furazolidine Nifuxoxazide Nifuratel-used in vet. medicine Nitrofurazole (was used for topical application ears, eye vagina Nitrofulantion causes a lot of vomiting so pts do not take them. Nifueatel is used for coccidiosis. Toxic effects Disulfuram like effects GIT disturbance manifested as nausea, vomiting, diarrheoa Hypersensitivity reactions skin each, drug fever, in elderly pts there is a dramatic onset of chills and fever. [Show More]
Last updated: 1 year ago
Preview 1 out of 12 pages

Reviews( 0 )
Document information
Connected school, study & course
About the document
Uploaded On
Feb 10, 2021
Number of pages
12
Written in
Additional information
This document has been written for:
Uploaded
Feb 10, 2021
Downloads
0
Views
75