Pharmacology > Class Notes > cephalosprins (All)
cephalosprins
Document Content and Description Below
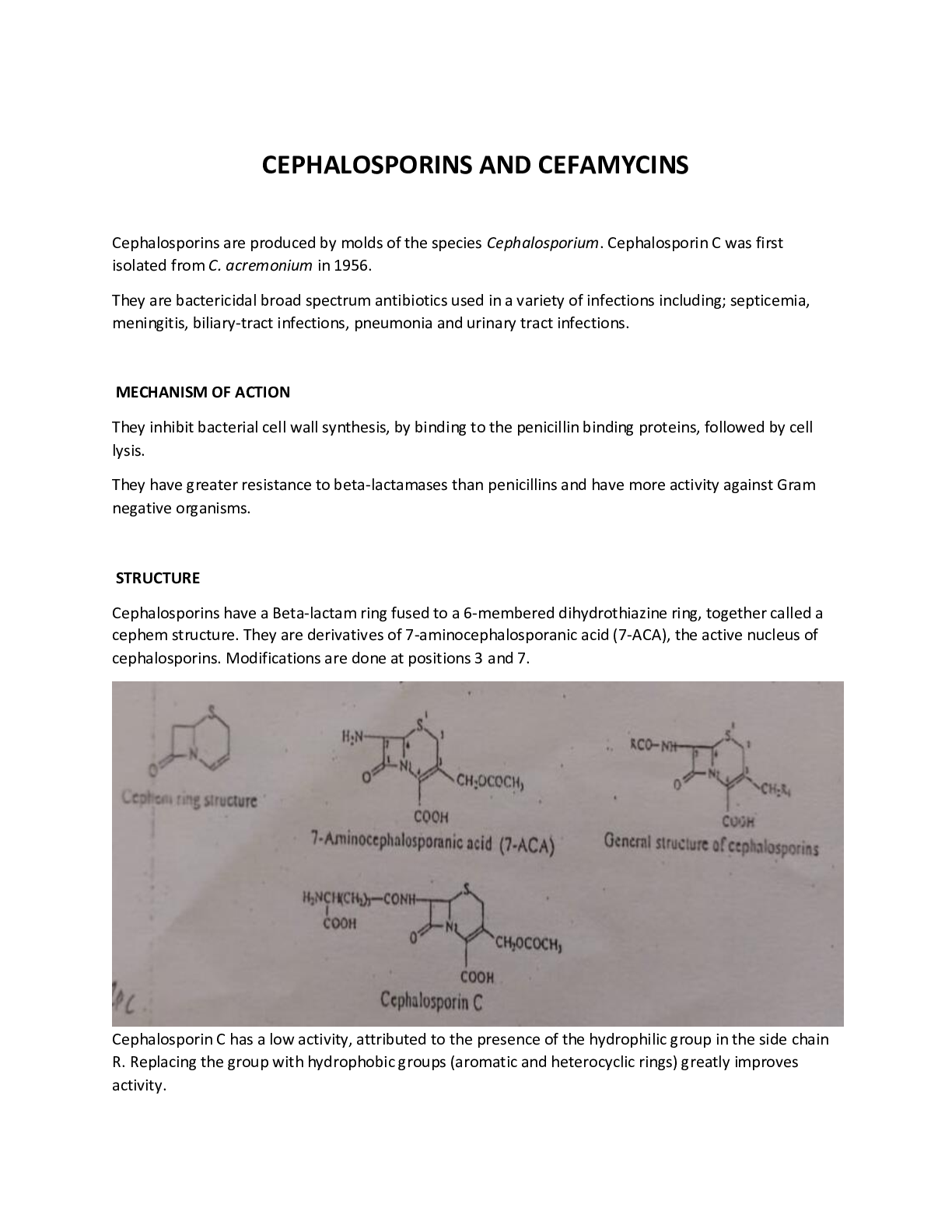
CEPHALOSPORINS AND CEFAMYCINS Cephalosporins are produced by molds of the species Cephalosporium. Cephalosporin C was first isolated from C. acremonium in 1956. They are bactericidal broad spectrum... antibiotics used in a variety of infections including; septicemia, meningitis, biliary-tract infections, pneumonia and urinary tract infections. MECHANISM OF ACTION They inhibit bacterial cell wall synthesis, by binding to the penicillin binding proteins, followed by cell lysis. They have greater resistance to beta-lactamases than penicillins and have more activity against Gram negative organisms. STRUCTURE Cephalosporins have a Beta-lactam ring fused to a 6-membered dihydrothiazine ring, together called a cephem structure. They are derivatives of 7-aminocephalosporanic acid (7-ACA), the active nucleus of cephalosporins. Modifications are done at positions 3 and 7. Cephalosporin C has a low activity, attributed to the presence of the hydrophilic group in the side chain R. Replacing the group with hydrophobic groups (aromatic and heterocyclic rings) greatly improves activity. Cefamycins have a methoxy group or its derivatives attached to C-7 instead of a hydrogen atom and are more resistance to hydrolysis by B-lactamases. PHYSICOCHEMICAL PROPERTIES The cephem ring structure of cephalosporins is much less strained than the penam structure of penicillins which renders them less reactive and less potent. The double bond at C 3,4 and the electron withdrawing groups at C-3 introduced in semisynthetic compounds compensate for this lack of reactivity. Cephalosporins can be inactivated by penicillinases through breakup of the beta-lactam ring. There are less common incidences of allergy associated with cephalosporins compared to penicillins. Being carboxylic acids, cephalosporins form water soluble salts with inorganic bases and injectable forms are often sodium salts. SYNTHESIS 7-ACA, obtained by cleavage of the side chain of natural Cephalosporin C is the starting point of synthesis of cephalosporins. The penicillin nucleus may also be converted to the cephalosporin nucleus through an elaborate chemical process. Semi-synthesis is done with the aim of; ● Increasing acid stability ● Improving antibacterial activity ● Imparting oral activity Substitutions at position 7 mainly alter antibacterial activity while those at position 3 affect metabolism and other pharmacokinetic parameters. METABOLISM The acetyl group at position 3, where present, is hydrolyzed to less active alcohols. The alcohol group then rapidly forms a cyclic ester (lactone) with the carboxylic group on the adjacent C atom. The lactones are inactive. Compounds without the acetyl group are mainly excreted unchanged in urine. STRUCTURE ACTIVITY RELATIONSHIP The addition of an amino and a hydrogen to the α and α′ position, respectively, results in a basic compound that is protonated under the acidic conditions of the stomach. The ammonium ion improves the stability of the β-lactam of the cephalosporin, leading to orally active drugs. E.g. Cephalexin, cefadroxil, Cefaclor, Cefprozil. The 7β amino group is essential for antimicrobial activity (X = H), whereas replacement of the hydrogen at C-7 (X = H) with an alkoxy (X = OR) results in improvement of the antibacterial activity of the cephalosporin. E.g. Cefoxitin, Cefotetan and Cefmetazole (2 Generation –OCH3) Within specific cephalosporin derivatives, the addition of a 7α methoxy also improves the drugs stability toward β-lactamase. E.g. Cefuroxime. The derivatives where Y = S exhibit greater antibacterial activity than if Y = O, but the reverse is true when stability toward β-lactamase is considered. The 6α hydrogen is essential for biological activity. Antibacterial activity is improved when Z is a 5-membered heterocycle versus a 6-membered heterocycle. E.g Ceftriazone, Ceftazidime, Cefditoren pivoxil (3-Generation) CLASSIFICATION 1. FIRST GENERATION CEPHALOSPORINS i. Parenteral: Cephapirin, Cephalothin, Cephazolin. ii. Oral and parenteral: Cephadrine iii. Oral: Cephalexin, Cefadroxil These agents are very active against gram-positive bacteria, but have only modesty against gramnegative bacteria. They’re not active against methicillin-resistance Staphylococcus aureus (MRSA) and have poor activity against Pseudomonas aeruginosa. 2. SECOND GENERATION CEPHALOSPORINS i. Parenteral agents: Cefamandole, Cefoxitin ii. Oral and parenteral: Cefuroxime (Zinnat-Oral, Zinacet-IV) iii. Oral: Cefaclor, Cefprozil, Loracarbef These agents have activity against many microorganisms that are resistant to the first generation cephalosporins. They also have more activity against gram-negative bacteria with less activity against gram-positive bacteria. They have poor activity against P.aeroginosa. 3. THIRD GENERATION CEPHALOSPORINS They are effective against a wider spectrum of gram negative organisms and are able to cross blood brain barrier (maybe used to treat menengitis). The nitrogen of heterocyclic rings at C3 gives the compounds good aqueous stability and gram-negative activity. Examples: i. Parenteral agents: Cefotaxime, Ceftriazone, Ceftazidime, Ceftizoxime, Cefepime ii. Oral agents: Cefixime, Cefdinir, Cefpodoxime, Ceftibuten, Cefditoren Pivoxil. 4. FOURTH GENERATION CEPHALOSPORINS Examples: Cefepime, Cefquinome, Cefpirome, Cefozopran, Cefluprenam Cefepime is a semisynthetic agent containing a Z-methoxyimine moiety and an aminothiazolyl group at C-7, broadening its spectrum and increasing its β-lactamase stability as well as increasing its antistaphylococcal activity. The quaternary N-methylpyrrolidine group at C-3 seems to help penetration into Gram-negative bacteria. The fourth-generation Cephalosporins are characterized by enhanced antistaphylococcal activity and broader anti-Gram-negative activity than the third-generation group. Cefepime is used IM and IV against urinary tract infect ions, skin and skin structure infections, pneumonia, and intra-abdominal infect ions. (Structure of Cefepime) 5. FIFTH GENERATION CEPHALOSPORINS They exhibit broad-spectrum activity against gram-positive bacteria, including MRSA and extensivelyresistant strains, such as Vancomycin-resistant S. aureus. Examples: Ceftaroline, Ceftolozane, Ceftobiprole, THIENAMYCINS They’ve a carbapenem nucleus consisting of a Beta-lactam ring fused to a pyrroline ring. They have the broadest spectrum of activity of the antibiotics. The prototype is thienamycin. The presence of a C atom at position 4, (instead of S as in penicillins), together with the group at position 3 and the double bond at C 2-3 make thienamycin very reactive and very potent, which also makes it chemically unstable. Chemical instability is improved by replacing the group at C3 with less reactive group. E.g. Imipenem and Meropenem. (Structures of carbapenem and thienamycin) Imipenem is active against both gram-positive and gram-negative bacteria. Renal dehydropeptidase inactivates Imipenem through hydrolysis, and its therefore formulated with Cilastin, a dehydropeptidase inhibitor to counteract this effect. Meropenem is effective as a single agent. (Structures of Imipenem, Meropenem, Cilastin) MONOBACTAMS These are monocyclic Beta-lactam antibiotics produced by various molds. The semi-synthetic agent Aztreonam is very active against gram-negative bacteria and inactivates beta-lactamases. Used for Pseudomonas infection. (Structure of Aztreonam ) NORCARDICINS Norcardicins are monocyclic Beta-lactam antibiotics with a broad spectrum of activity but with low intrinsic activity. (Structure of Norcadicin A) BETA-LACTAMASE INHIBITORS Includes: Clavulanic Acid, Sulbactam, Tazobactam. (Structures of Clavulanic Acid,Sulbactam, Tazobactam) [Show More]
Last updated: 1 year ago
Preview 1 out of 9 pages
Instant download
Instant download
Reviews( 0 )
Document information
Connected school, study & course
About the document
Uploaded On
Feb 10, 2021
Number of pages
9
Written in
Additional information
This document has been written for:
Uploaded
Feb 10, 2021
Downloads
0
Views
89