SCIENCE 101 > GIZMOS > Student Exploration: Collision Theory Gizmo Rated A (All)
Student Exploration: Collision Theory Gizmo Rated A
Document Content and Description Below
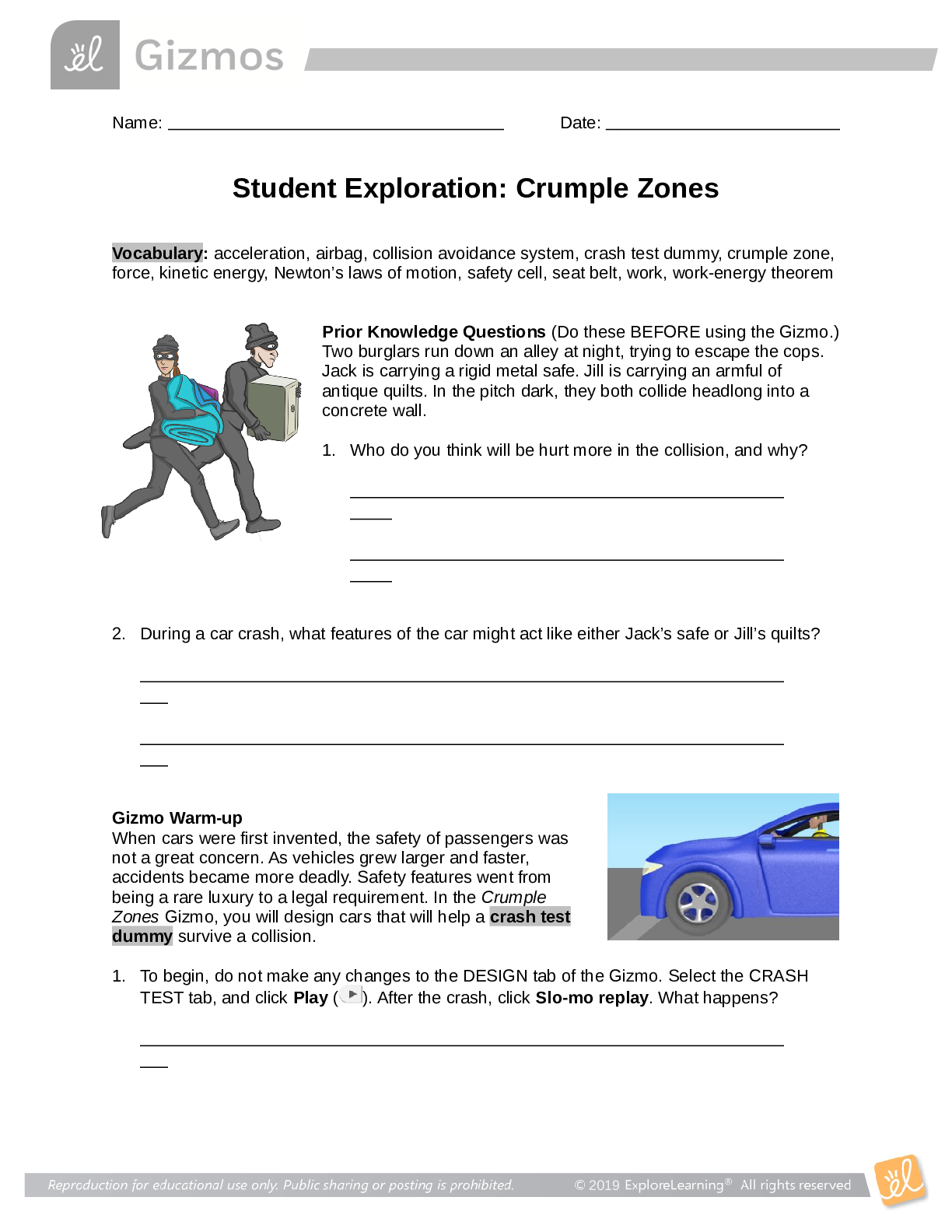
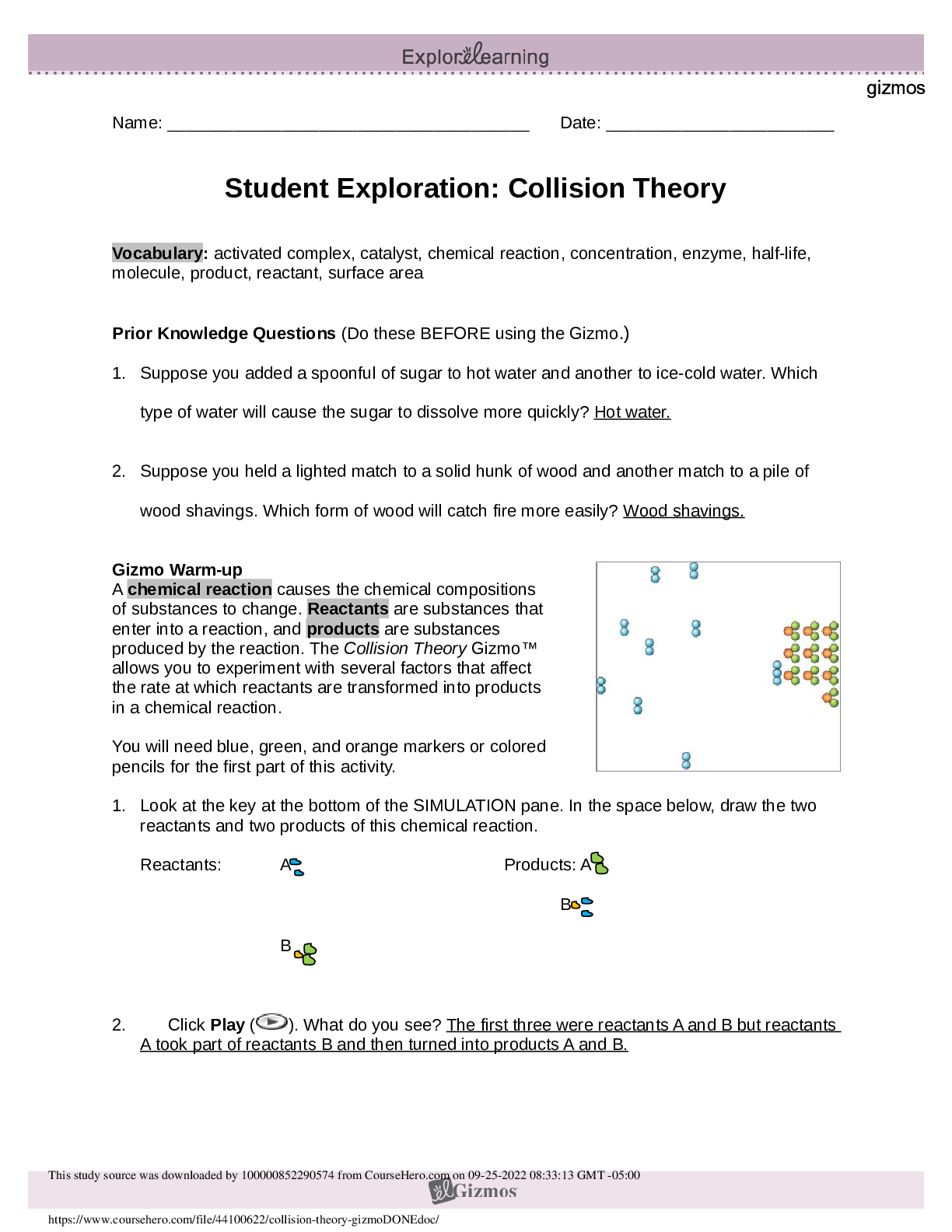
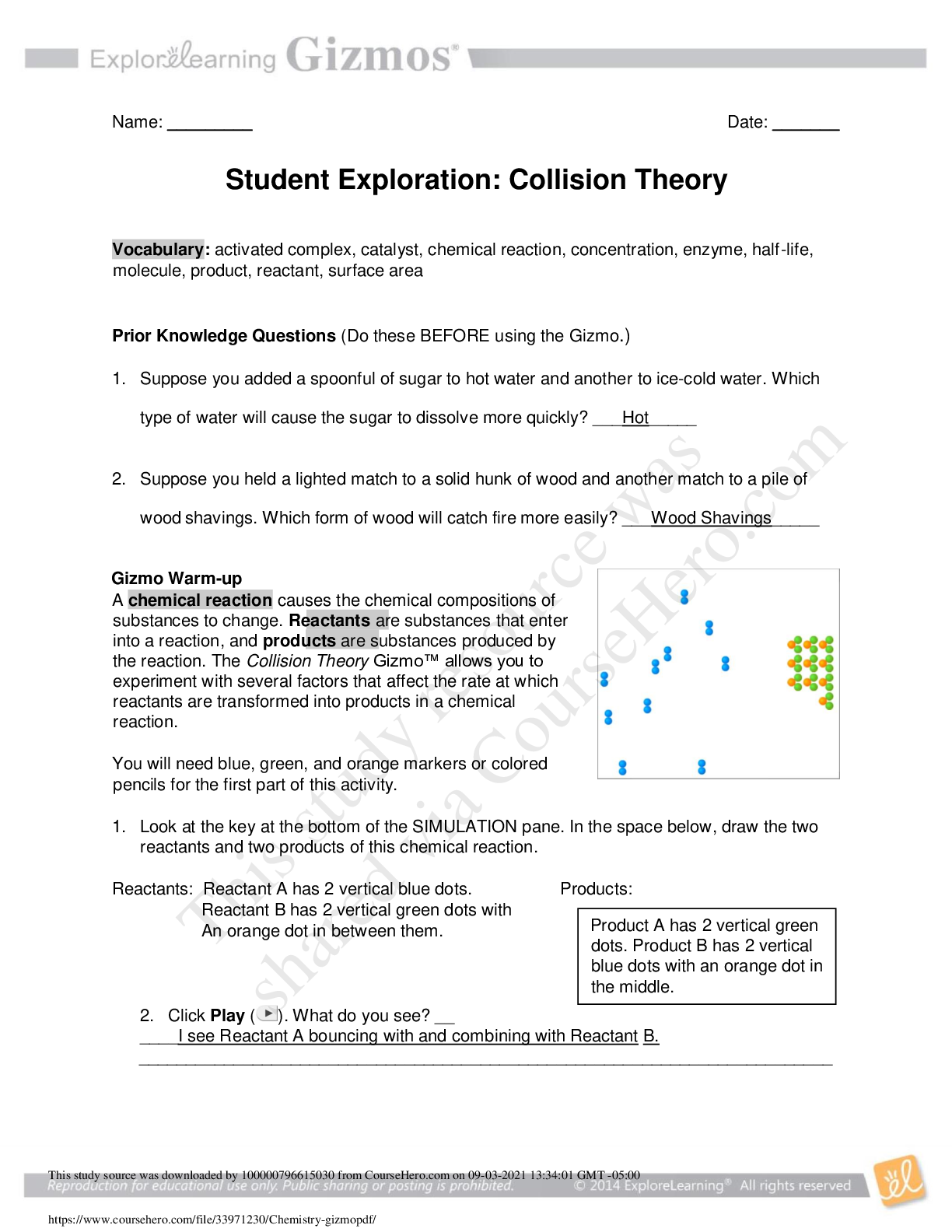
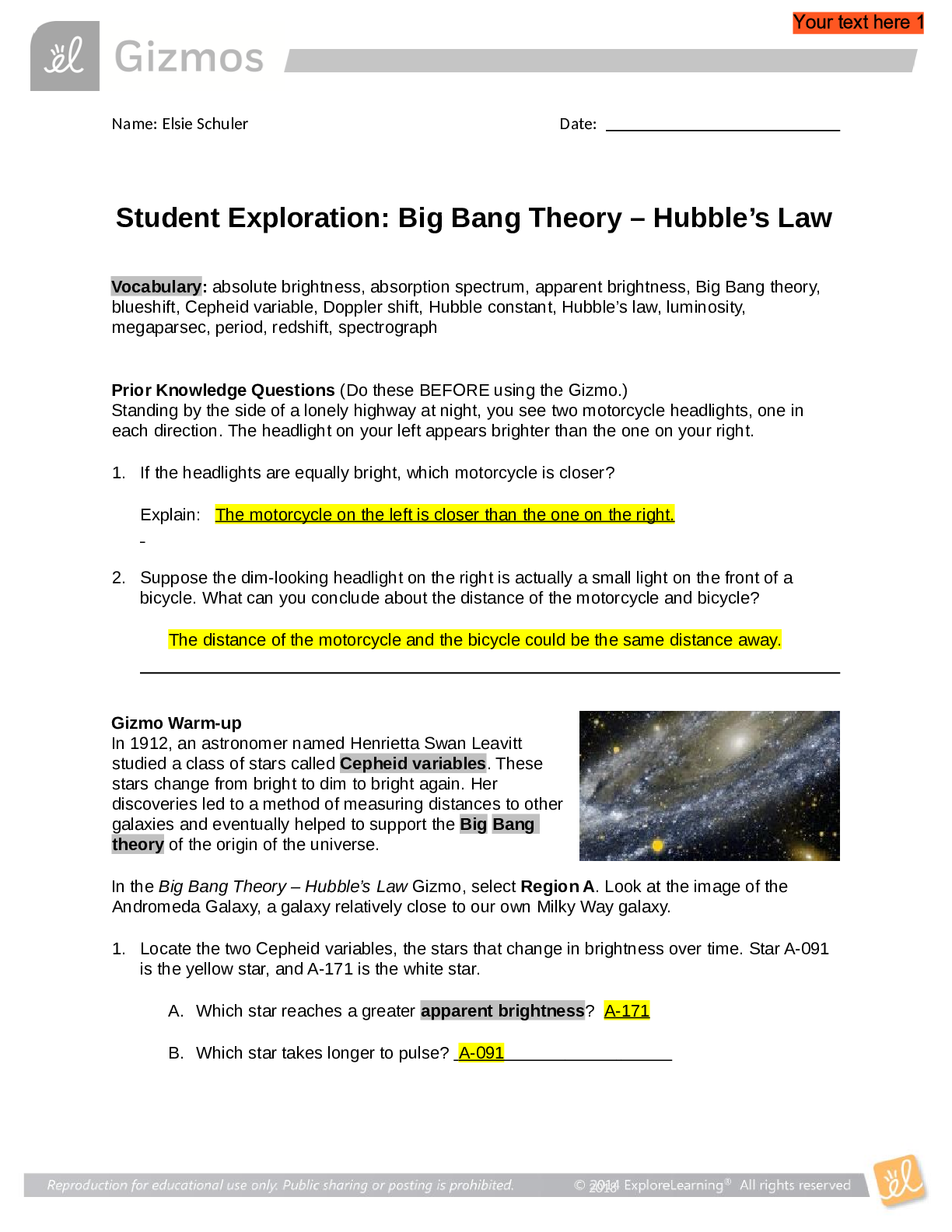
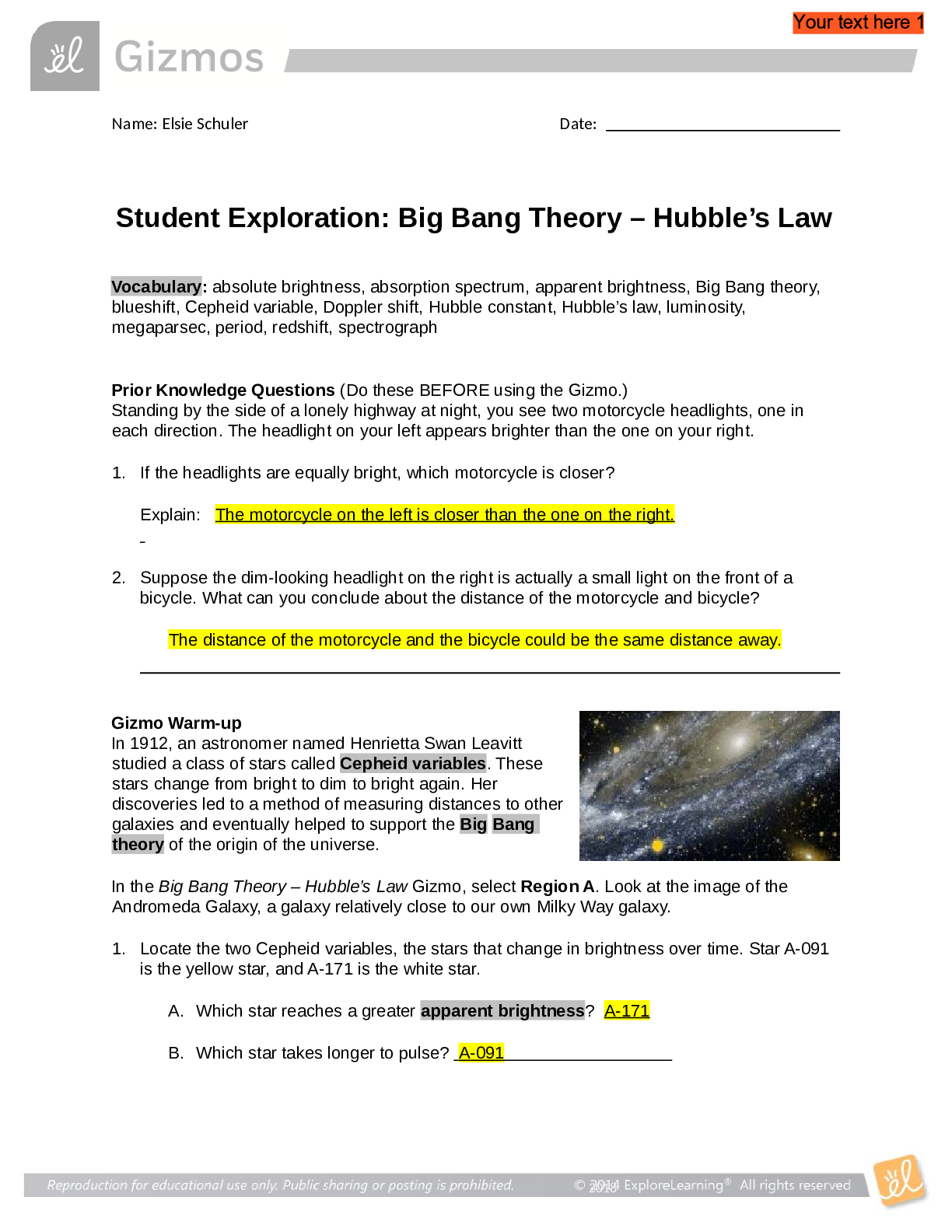
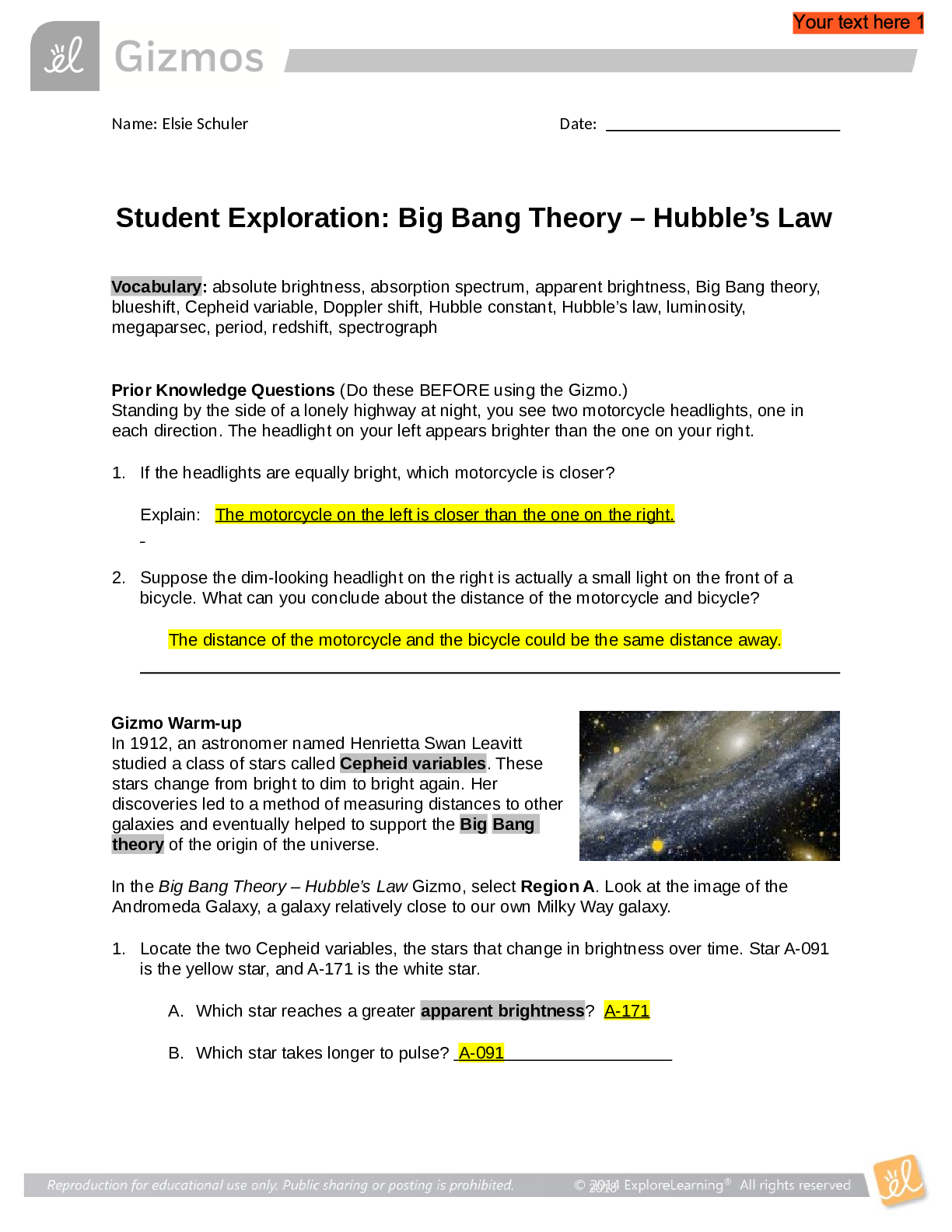
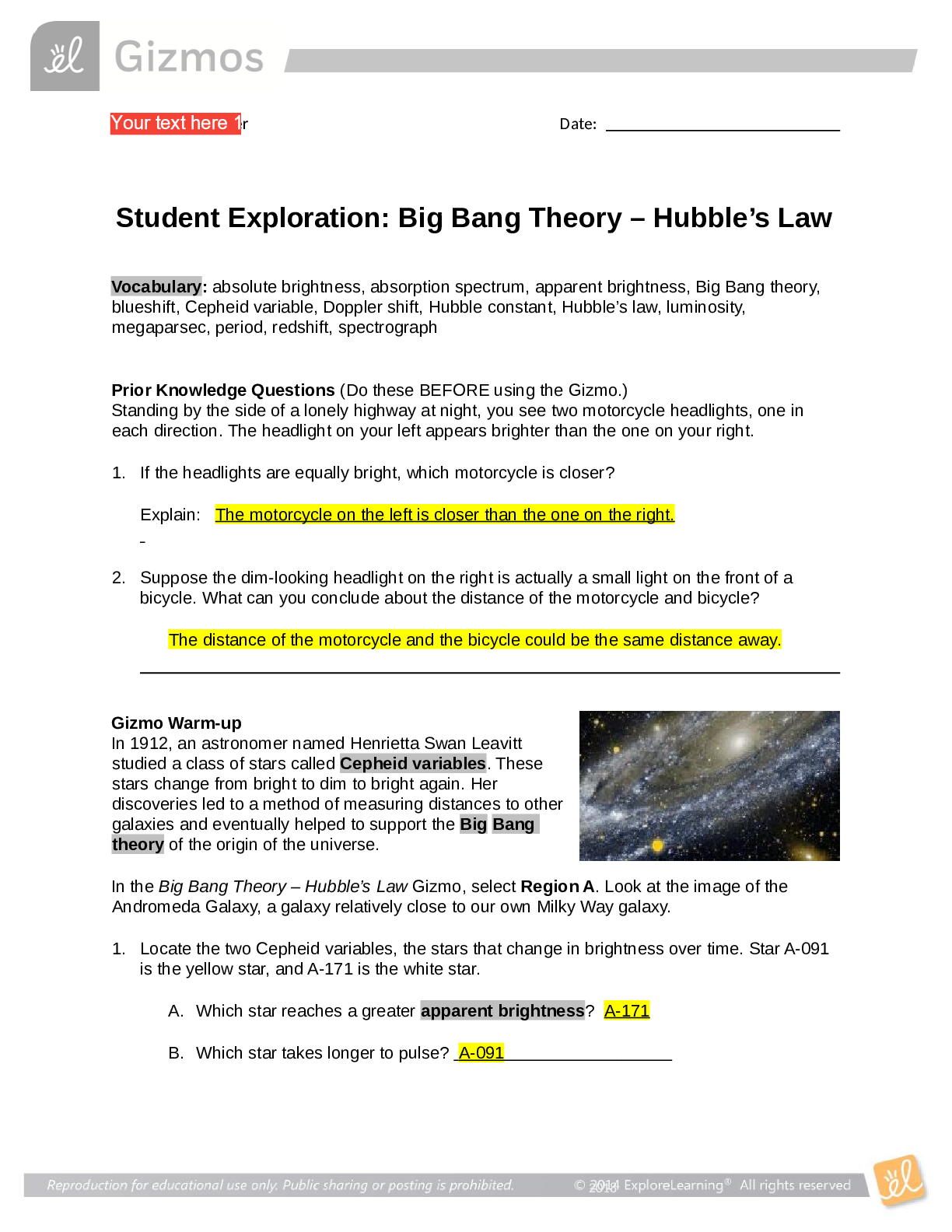
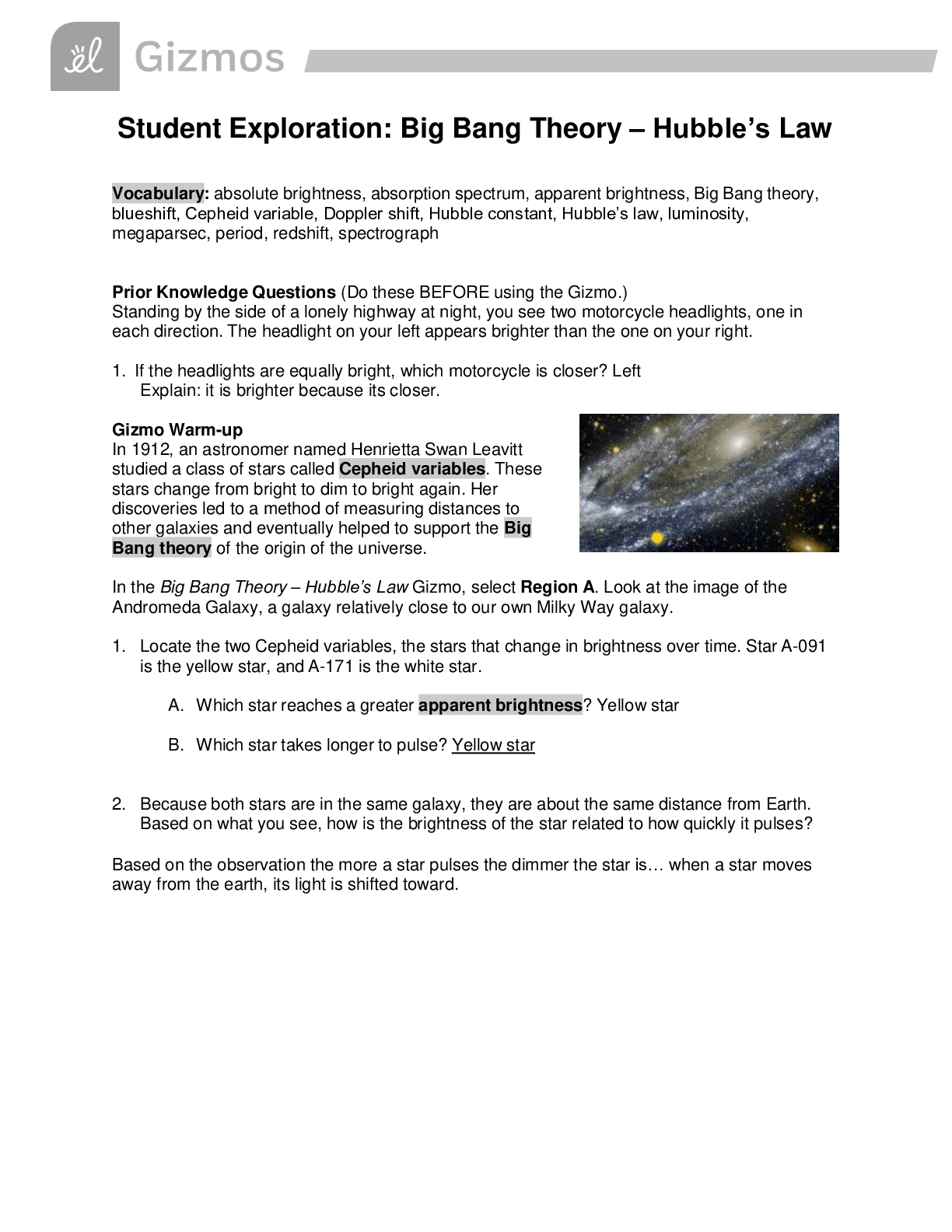
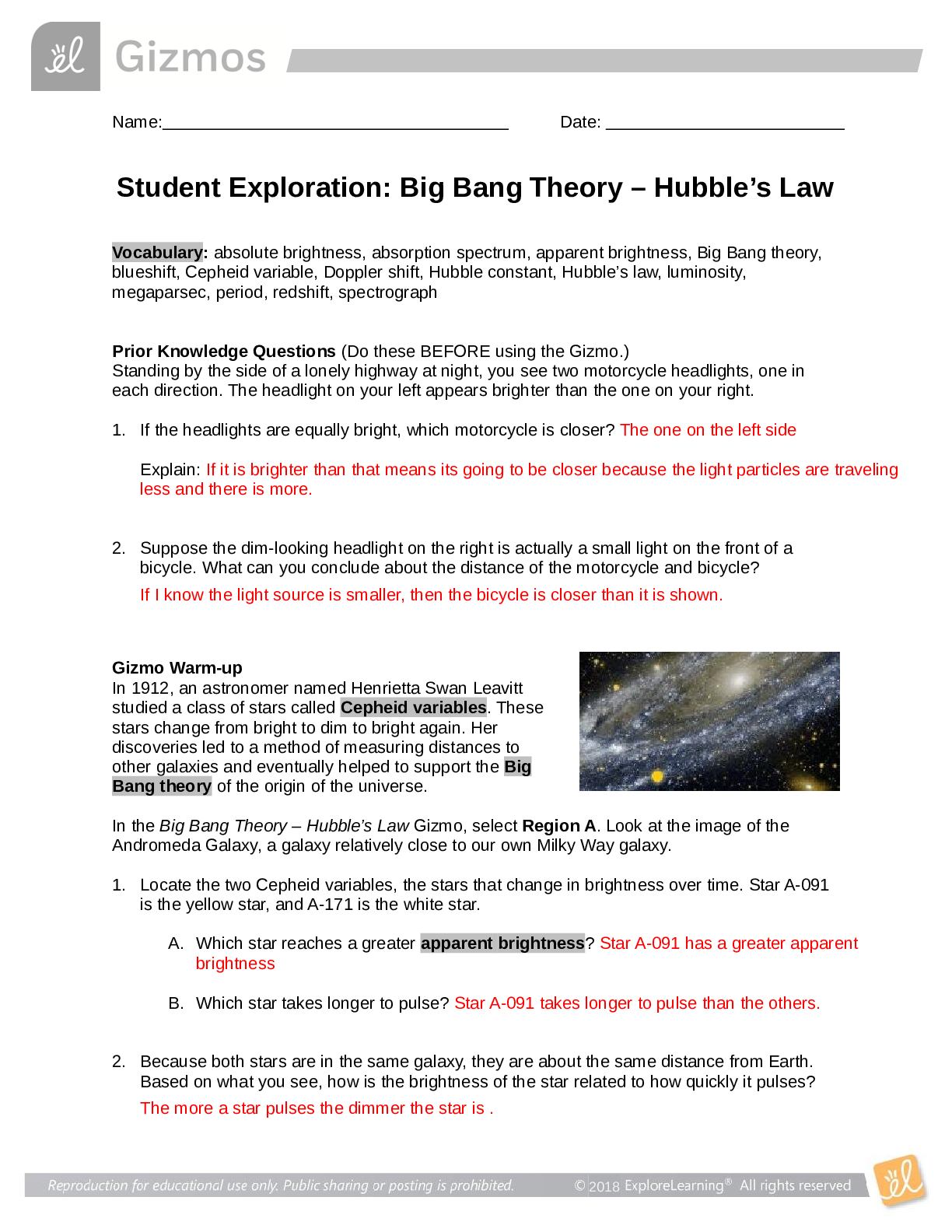
Vocabulary: activated complex, catalyst, chemical reaction, concentration, enzyme, half-life, molecule, product, reactant, surface area Prior Knowledge Questions (Do these BEFORE using the Gizmo.) ... 1. Suppose you added a spoonful of sugar to hot water and another to ice-cold water. Which type of water will cause the sugar to dissolve more quickly? Hot 2. Suppose you held a lighted match to a solid hunk of wood and another match to a pile of wood shavings. Which form of wood will catch fire more easily? Shavings Gizmo Warm-up A chemical reaction causes the chemical compositions of substances to change. Reactants are substances that enter into a reaction, and products are substances produced by the reaction. The Collision Theory Gizmo™ allows you to experiment with several factors that affect the rate at which reactants are transformed into products in a chemical reaction. You will need blue, green, and orange markers or colored pencils for the first part of this activity. 1. Look at the key at the bottom of the SIMULATION pane. In the space below, draw the two reactants and two products of this chemical reaction. Reactants: Products: 2. Click Play ( ). What do you see? Reactants moving around and combing to form the products This study source was downloaded by 100000796615030 from CourseHero.com on 09-03-2021 13:26:40 GMT -05:00 https://www.coursehero.com/file/21134166/Collision-Theory-Notes/ This study resource was shared via CourseHero.com Activity A: Temperature Get the Gizmo ready: Click Reset ( ). Check that the Reactant concentration is set to 1.0 mol/L, the Catalyst concentration is set to 0.00 mol/L, and the Surface area is Minimum. Question: How does temperature affect the rate of a chemical reaction? 1. Observe: Select the ANIMATION tab. View the animation with No catalyst selected. What do you see? The two come together, transfer the central atom, and separate When two reactant molecules meet, they form a temporary structure called an activated complex. The activated complex breaks up into the product molecules. 2. Observe: Return to the CONTROLS pane. Set the Temperature to 0 °C and the Simulation speed to its maximum setting. Click Play. A. Describe the motions of the molecules. Reactant A moves around but doesn’t combine with Reactant B B. Now set the Temperature to 200 °C. How does increasing the temperature affect the motions of the molecules? The two reactants move and combine very fast C. What do you notice about the chemical reaction at the higher temperature? It happens faster 3. Interpret: Select the GRAPH tab. Click the zoom out button (–) until you can see the whole graph. What does this graph show? Concentration of reactants/products 4. Predict: How do you think temperature will affect the rate of a chemical reaction? Higher temperature increases the rate of a chemical reaction (Activity A continued on next page) This study source was downloaded by 100000796615030 from CourseHero.com on 09-03-2021 13:26:40 GMT -05:00 https://www.coursehero.com/file/21134166/Collision-Theory-Notes/ This study resource was shared via CourseHero.com Activity A (continued from previous page) 5. Gather data: Click Reset. A useful way to compare reaction rates is to record the time required for half of the reactants to react, called the half-life of the reaction. With the Temperature set to 200 °C, click Play. Click Pause ( ) when the number of reactant molecules is 10. Record the half-life time in the first space of the table below. Trial 200 °C 150 °C 100 °C 50 °C 1 04:16 10:06 37:34 70:14 2 08:58 04:12 06:50 103:20 Mean half-life 06:01 07:18 22:20 87:18 Repeat the experiment at different temperatures to complete the table. (Note: To get exact times, you can refer to the TABLE tab.) [Show More]
Last updated: 1 year ago
Preview 1 out of 7 pages
.png)
Reviews( 0 )
Document information
Connected school, study & course
About the document
Uploaded On
Sep 03, 2021
Number of pages
7
Written in
Additional information
This document has been written for:
Uploaded
Sep 03, 2021
Downloads
0
Views
119







.png)








.png)

